JAC Board Class 9th Science Solutions Chapter 4 Structure of the Atom
JAC Class 9th Science Structure of the Atom InText Questions and Answers
Page 47
Question 1.
What are canal rays?
Answer:
Canal rays are radiations which are positively charged. They were the key in the discovery of proton, another positively charged sub – atomic particle.
Question 2.
If an atom contains one electron and one proton, will it carry any charge or not?
Answer:
Since an electron is a negatively charged particle and the proton, a positively charged one, the net charge becomes neutral as both particles neutralise each
Page 48
Question 1.
On the basis of Thomson’s model of an atom, explain how the atom is neutral as a whole.
Answer:
According to Thomson’s model of an atom:
- an atom consists of a positively charged sphere in which the negatively charged electrons are embedded.
- the number of protons and electrons are equal in an atom, thereby, neutralising their charge keeping the overall system neutral.
Question 2.
On the basis of Rutherford’s model of an atom, which sub-atomic particle is present in the nucleus of an atom?
Answer:
As per Rutherford’s model of atom, the positively charged protons are the ones that are present in the nucleus of an atom.
![]()
Question 3.
Draw a sketch of Bohr’s model of an atom with three shells.
Answer:
Bohr’s model of an atom with three shells:
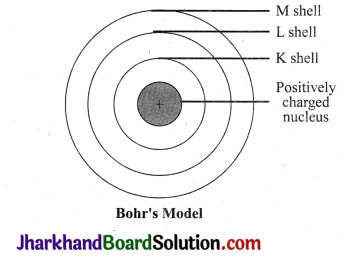
Question 4.
What do you think would be the observation if the α – particle scattering experiment is carried out using a foil of a metal other than gold?
Answer:
When any other metal foil is used instead of gold, the observation would remain the same. This is because the structure of an atom, when considered individually, remains the same.
Page 49
Question 1.
Name the three subatomic particles of an atom.
Answer:
An atom consists of three subatomic particles:
- Protons : Positively charged
- Electrons : Negatively charged
- Neutrons : Neutral in nature (no charge)
Question 2.
Helium atom has an atomic mass of 4u and two protons in its nucleus. How many neutrons does it have?
Answer:
Atomic mass of He = 4u
Atomic mass = number of (protons + neutrons)
4 = 2 + number of neutrons Number of neutrons = 4 – 2
= 2 Helium atom has 2 neutrons.
Page 50
Question 1.
Write the distribution of electrons in carbon and sodium atoms.
Answer:
1. Carbon atom:
Atomic number = 6
Number of protons = 6 = Number of electrons
Distribution of electrons = K – 2, L – 4.
2. Sodium atom:
Atomic number = 11
Number of protons = 11 = Number of electrons
Distribution = K – 2, L – 8, M – 1.
Question 2.
If K and L shells of an atom are full, then what would be the total number of electrons in the atom?
Answer:
Number of electrons K shell can hold = 2 Number of electrons L shell can hold = 8
Hence, when both the shells are full, the total number of electrons present = 2 + 8 ⇒ 10 electrons.
Page 52
Question 1.
How will you find the valency of chlorine, sulphur and magnesium?
Answer:
Valency is the combining capacity of the atom of an element.
1. Chlorine: Atomic number = 17
Number of protons = Number of electrons = 17
Distribution: K – 2, L – 8, M – 7 Chlorine needs 1 electron to complete its outermost orbit shell. Its valency is – 1 (gains 1 electron).
2. Sulphur: Atomic number = 16 Number of protons = Number of electrons = 16
Distribution: K – 2, L – 8, M – 6 Sulphur needs 2 electrons to complete its outermost shell. Its valency is – 2 (gains 2 electrons).
3. Magnesium: Atomic number = 12
Number of protons = Number of electrons = 12
Distribution: K – 2, L – 8, M – 2 Magnesium needs to donate 2 electrons from its outermost shell to become stable. Its valency is + 2 (donate 2 electrons).
Question 2.
If the number of electrons in an atom is 8 and number of protons is also 8, then
(a) what is the atomic number of the atom?
(b) what is the charge on the atom?
Answer:
Number of electrons = 8, Number of protons = 8
(a) Atomic number of the atom = Number of protons = 8
(b) As the number of electrons is equal to the number of protons on the atom, their charges neutralise each other. Therefore, the atom does not possess any charge.
![]()
Question 3.
With the help of the table 4.1, find out the mass number of oxygen and sulphur atom.
Answer:
- Oxygen: Number of protons = 8 Number of neutrons = 8 Atomic number = 8
Mass number = Number of (protons + neutrons) = 8 + 8 = 16u. - Sulphur: Number of protons = 16 Number of neutrons = 16 Atomic number = 16
Mass number = Number of (protons + neutrons) = 16 + 16 = 32u.
Page 53
Question 1.
For the symbols H, D and T, tabulate three subatomic particles found in each of them.
Answer:
The symbols H, D and T, tabulate three subatomic particles:
| Element | H (Protium) (11H) | D (Deute – rium) (2H) | T (Tritium) (31H) |
| Number of protons | 1 | 1 | 1 |
| Number of electrons | 1 | 1 | 1 |
| Number of neutrons | Nill | 1 | 2 |
Question 2.
Write the electronic configuration of any one pair of isotopes and isobars.
Answer:
(a) Isotopes : Isotopes are atoms which have the same number of protons but the number of neutrons differs. This leads to the variation in mass number too.
Example :
The simplest example is the carbon molecule which exists as \({ }_{6} \mathrm{C}^{12}\) and \({ }_{6} \mathrm{C}^{14}\) but when their electronic configuration is noticed, both have K – 2, L – 4.
(b) Isobars : Isobars are the atoms having the same mass number but differ in the atomic numbers. Electronic configuration of an isobar pair is as follows:
Example :
40Ca20: K – 2, L – 8, M – 8, N – 2
40Ar18 : K – 2, L – 8, M – 8
JAC Class 9th Science Atoms and Molecules Textbook Questions and Answers
Question 1.
Compare the properties of electrons, protons and neutrons.
Answer:
The properties of electrons, protons and neutrons:
| Electrons | Protons | Neutrons |
| Negatively charged | Positively charged | No charge |
| Present outside the nucleus | Present within the nucleus | Present inside the nucleus of an atom |
| Negligible mass | 1 a.m.u. | 1 a.m.u. |
| Get attracted towards positive electrode | Get attracted towards negative electrode | Do not get attracted to any charged particle. |
Question 2.
What are the limitations of J.J. Thomson’s model of an atom?
Answer:
According to J.J. Thomson’s model of an atom, the electrons are embedded all over in the positively charged sphere. But experiments done by the other scientists show that protons are present only in the centre of the atom and electrons are distributed around it.
Question 3.
What are the limitations of Rutherford’s model of atom?
Charged bodies, when move in circular motion, emit radiations. Thus, electrons revolving round the nucleus, as suggested by Rutherford, will lose energy and will come closer and closer to the nucleus and will finally merge into the nucleus. This means that atoms are quite unstable which is not true.
![]()
Question 4.
Describe Bohr’s model of atom.
Answer:
(a) The nucleus of an atom is present in the centre.
(b) Negatively charged electrons revolve around this nucleus.
(c) Discrete orbits of electrons are present inside the atom.
(d) While revolving in the orbit, the electrons do not radiate energy.
(e) These discrete orbits are represented as K, L, M, N orbits or denoted by
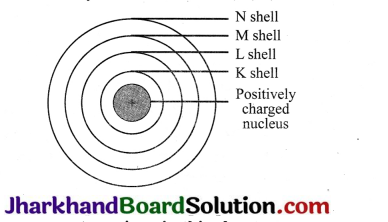
Question 5.
Compare all the proposed models of an atom given in this chapter.
Answer:
| Thomson’s atomic model | Rutherford’s atomic model | Bohr’s atomic model |
| Sphere of positive charge | Sphere of positive charge in centre is called nucleus. All mass of an atom resides in the nucleus. | Positive charge in centre is called nucleus. |
| Electrons are spread randomly all over in the sphere. | Electrons revolve around the nucleus in well defined orbits. | Electrons revolve in discrete orbits and do not radiate energy. |
| Positive charge = negative charge. | Size of nucleus is very small as compared to the size of atom. | The orbits were termed as energy shells |
| Atom is electricity – neutral. | Rutherford’s atomic model | labelled as K, L, M, N or n = 1,2, 3, 4. |
Question 6.
Summarise the rules for writing of distribution of electrons in various shells for the first 18 elements.
Answer:
(a) Generally, the maximum number of electrons that can be accommodated in a shell is given by the formula: 2n2, where n = 1, 2, 3 … .
(b) Maximum number of electrons in different shells are:
K shell (n = 1), 2n2 = 2(1)2 = 2
L shell (n = 2), 2n2 = 2(2)2 = 8
M shell (n = 3), 2n2 = 2(3)2 = 18
N shell (n = 4), 2n2 = 2(4)2 = 32.
(c) The electrons are not taken in unless the inner shells are completely filled.
Question 7
Define valency by taking examples of silicon and oxygen.
Answer:
Valency is the combining capacity of an atom. Take the examples of silicon and oxygen:
| Oxygen | Silicon |
| Atomic Number : 8 | Atomic Number : 14 |
| Electronic Config : K – 2, L – 6 | Electronic Config : K – 2, L – 8, M – 4 |
| Valence electrons : 6 | Valence electrons : 4 |
| Valency : 8 – 6 = 2 | Valency : 8 – 4 = 4 |
In the atoms of oxygen, the valence electrons are 6.
To fill the orbit, 2 electrons are required In the atom of silicon, the valence electrons are 4.
To fill this orbit 4 electrons are required Hence, the combining capacity of oxygen is 2 and of silicon is 4, i.e., valency of oxygen = 2 and valency of silicon = 4.
Question 8.
Explain with examples.
(a) Atomic number
(b) Mass number
(c) Isotopes
(d) Isobars
Give any two uses of isotopes.
Answer:
(a) Atomic number : The atomic number of an element is the total number of protons present in the atom of that element. For example, nitrogen has 7 protons in its atom. Thus, the atomic number of nitrogen is 7.
(b) Mass number : The mass number of an element is the. sum of the number of protons and neutrons present in the atom of that element. For example, the atom of boron has 5 protons and 6 neutrons. So, the mass number of boron is 5 + 6 =11.
(c) Isotopes : These are atoms of the same element having the same atomic number, but different mass numbers. For example, chlorine has two isotopes with atomic number 17 but mass numbers 35 and 37 represented by \({ }_{17}^{35} \mathrm{Cl}\) \({ }_{17}^{37} \mathrm{Cl}\).
(d) Isobars : These are atoms having the same mass number but different atomic number, i.e, isobars are atoms of different elements having the same mass number. For example,
Ca has atomic number 20 and Ar has atomic number 18 but both of them have mass number 40 represented by \({ }_{20}^{40} \mathrm{Ca}\) and \({ }_{18}^{40} \mathrm{Ar}\) respectively.
Two uses of isotopes:
- An isotope of uranium is used as a fuel in nuclear reactors.
- An isotope of cobalt is used in the treatment of cancer.
![]()
Question 9.
Na+ has completely filled K and L shells. Explain.
Answer:
The atomic number of sodium is 11. So, neutral sodium atom has 11 electrons and its electronic configuration is 2, 8, 1. But Na+ has 10 electrons. Out of 10, K – shell contains 2 and L – shell has 8 electrons. Thus, Na+ has completely filled K and L shells.
Question 10.
If bromine atom is available in the 79 form of, say, two isotopes \({ }_{35}^{79} \mathrm{Br}\) (49.7%) and \({ }_{35}^{81} \mathrm{Br}\) (50.3%), calculate the average atomic mass of bromine atom.
Answer:
The atomic masses of two isotopic atoms are 79 (49.7%) and 81 (50.3%).
Thus, total mass = (79 × \(\frac{49.7}{100} \) ) + (81 × \(\frac{50.3}{100} \)) = 39.263 + 40.743 = 80.006u.
Question 11.
The average atomic mass of a sample of an element X is 16.2 u. What are the percentages of isotopes \({ }_{8}^{16} \mathrm{X}\) and \({ }_{8}^{18} \mathbf{X}\) in the sample?
Answer:
It is given that the average atomic mass of the sample of element X is 16.2 u. Let the % of isotope \({ }_{8}^{16} \mathrm{X}\) be y%. Thus, the % of isotopes \({ }_{8}^{18} \mathbf{X}\) will be (100 – y) %. Therefore,
16 × \(\frac{\mathrm{y}}{100}\) + \(\frac{18 \times(100-y)}{100}\) = 16.2
\(\frac{16 y}{100}\) + \(\frac{18(100-y)}{100}\) = 16.2
\(\frac{16 y+1800-18 y}{100}\) = 16.2
1800 – 2y = 1620 or 2y = 1800 – 1620 = y – 90
Therefore, the % of isotope \({ }_{8}^{16} \mathrm{X}\) is 90%.
And, the % of the isotope \({ }_{8}^{18} \mathbf{X}\) is (100 – 90) % = 10%.
Question 12.
If Z = 3, what would be the valency of the element? Also, name the element.
Answer:
Z = atomic number = 3 (given) Electronic configuration = K – 2, L – 1 Thus, valency = 1 The element with atomic number 3 is lithium.
Question 13.
The composition of the nuclei of two atomic species X and Y are given as under
| X | Y | |
| Protons | 6 | 6 |
| Neutrons | 6 | 8 |
Give the mass number of X and Y. What is the relation between the two species?
Answer:
Mass number of X = Protons + Neutrons = 6 + 6 = 12
Mass number of Y = Protons + Neutrons = 6 + 8 = 14
Since the atomic numbers of both the species are the same, they are the same element. Also, since they have different number of neutrons, their mass number is different and they are the isotopes.
Question 14.
For the following statements, write T for true and F for false.
(a) J. J. Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
(c) The mass of an electron is about 1/2000 times that of proton.
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
Answer:
(a) False
(b) False
(c) True
(d) False
Put tick against correct choice and cross against wrong choice in questions 15, 16 and 17.
Question 15.
Rutherford’s alpha – particle scattering experiment was responsible for the discovery of:
(a) Atomic nucleus
(b) Proton
(c) Electron
(d) Neutron
Answer:
(a) Atomic nucleus.
Question 16.
Isotopes of an element have:
(a) the same physical properties
(b) different number of neutrons
(c) different number of protons
(d) different atomic number
Answer:
(b) different number of neutrons.
Question 17.
Number of valence electrons in Cl– ion are:
(a) 16
(b) 8
(c) 17
(d) 18
Answer:
(b) 8.
Question 18:
Which one of the following is a correct electronic configuration of sodium?
(a) 2, 8
(b) 8, 2, 1
(c) 2, 1, 8
(d) 2, 8, 1
Answer:
(d) 2, 8, 1.
![]()
Question 19.
Complete the following table.
| Atomic number | Mass number | Number of neutrons |
| 9 | – | 10 |
| 16 | 32 | – |
| – | 24 | – |
| – | 2 | – |
| – | 1 | 0 |
| Number of protons | Number of electrons | Name of the atomic species |
| – | – | – |
| 12 | – | Sulphur |
| 1 | – | – |
Answer:
| Atomic number | Mass number | Number of neutrons |
| 9 | 19 | 10 |
| 16 | 32 | 16 |
| 12 | 24 | 12 |
| 1 | 2 | 1 |
| 1 | 1 | 0 |
| Number of protons | Number of electrons | Name of the atomic species |
| 9 | 9 | Fluorine |
| 16 | 16 | Sulphur |
| 12 | 12 | Magnesium |
| 1 | 1 | Hydrogen |
| 1 | 0 | Deuterium |
| 1 | 1 | Hydrogen |
| 1 | 0 | Protium |