JAC Board Class 9th Science Important Questions Chapter 3 Atoms and Molecules
Multiple Choice Questions
Question 1.
The value 6.022 × 1023 is also called:
(a) Dalton number
(b) Avogadro number
(c) Atomic number
(d) Mass number
Answer:
(b) Avogadros number
Question 2.
The number of moles in 52g of He is:
(a) 4
(b) 13
(c) 52
(d) 1
Answer:
(b) 13
Question 3.
1 amu means:
(a) 1/2th mass of C – 12atoms
(b) Mass of C – 12atom
(c) Mass of O – 16atom
(d) Mass of hydrogen molecules
Answer:
(a) 1/2th mass of C – 12atoms.
![]()
Question 4.
Formula for magnesium chloride is:
(a) MgCl
(b) Mg2Cl
(C) MgCl2
(d) MgCl4
Answer:
(C) MgCl
Question 5.
Formula for aluminium oxide is:
(a) AlO
(b) AlO4
(c) AlO4
(d) Al2O3
Answer:
(d) Al2O3
Question 6.
The percentage of hydrogen in H2O molecule is:
(a) 5.55%
(b) 11.11%
(c) 4445%
(d) 88.89%
Answer:
(b) 11.11%
Question 7.
Which of the following is not correctly matched?
(a) Calcium – Ca
(b) Silver – Ag
(e) Gold – Au
(d) Copper – Co
Answer:
(d) Copper – Co
Question 8.
Which of the following will have maximum mass?
(a) 0.1 mole NH3
(b) 1022 atoms of carbon
(c) 1022 molecules of CO2
(d) 1g of Fe
Answer:
(a) 0.1 mole NH3
Question 9.
Which of the following satements is not true about an atom?
(a) Atoms are not able to exist independently.
(b) Atoms are the basic units from which molecules and ions are formed.
(C) Atoms are always neutral in nature.
(d) Atoms aggregate in large numbers to form the matter that we can see, feel or touch.
Answer:
(a) Atoms are not able to exist independently.
Question 10.
Which of the following has maximum number of atoms?
(a) 18g of H2O
(b) 18g of O2
(c) 18g of CO2
(d) 18g of CH4
Answer:
(d) 18g of CH4
Question 11.
All noble gas molecules are
(a) monoatomic
(b) diatomic
(c) triatomic
(d) both (a) and (b)
Answer:
(a) monoatomic
Question 12.
The Valency of nitrogen in NH3 is?
(a) 1
(b) 3
(C) 4
(d) 5
Answer:
(b) 3
Question 13.
How many moles are present in 28g nitrogen atoms?
(a) 1 mole
(B) 2.3 moles
(c) 0.5 mole
(d) 2 moles
Answer:
(d) 2 moles
Question 14.
Molecules of which of the following elements are made up of only one atom of that element?
(a) Sodium
(b) Iron
(c) Helium
(d) Chlorine
Answer:
(c) Helium
![]()
Question 15.
Which of the following elements gives polyatomic molecules?
(a) Ne
(b) P
(e) Ni
(d) Si
Answer:
(b) P
Analysing & Evaluating Questions
Question 16.
Chemical formula of a metal sulphate is MSO4. What will be the formula of its chloride?
(a) MCl2
(b) MCl
(e) M2Cl
(d) M2Cl3
Answer:
(a) MCl2
Question 17.
Valenc of an clement X is 3. Write the chemical formula of its oxide.
(a) X2O3
(b) X2O
(c) XO2
(d) X3O2
Answer:
(a) X2O3
Question 18.
A silver ornament of mass ‘m’ gram is polished with gold equivalent to 1% of the mass of silver. Compute the ratio of the number of atoms of gold and silver in the ornament.
(a) 182.4 : 1
(b) 104 : 2
(c) 155 : 1
(d) 18 : 7
Answer:
(a) 182.4 : 1
Assertion Reason Questions
Directions:
In the following questions, the Assertions and the Reasons have been put forward. Read the statements carefully and choose the correct alternative from the following:
(A) Both the assertion and the reason are correct and the reason is the correct explanation of the assertion.
(B) The assertion and the reason are correct but the reason is not the correct explanation of the assertion.
(C) The assertion is true but the reason is false.
(D) Both the statements are false.
1. Assertion : Non – metals usually form anions.
Reason : Non – metals have the tendency to lose electrons.
Answer:
(C) The assertion is true but the reason is false.
2. Assertion : When a substance undergoes a chemical change, there is no change in total mass.
Reason : In a chemical substance, elements are always present in definite proportion by mass.
Answer:
(B) The assertion and the reason are correct but the reason is not the correct explanation of the assertion.
3. Assertion : Atoms cannot exist independently.
Reason : Atoms are the building blocks of elements.
Answer:
(B) The assertion and the reason are correct but the reason is not the correct explanation of the assertion.
4. Assertion : Carbon – 12 isotopes is taken as the standard element for measuring atomic mass.
Reason : Carbon lacks the tendency to form a large number of compounds.
Answer:
(C) The assertion is true but the reason is false.
5. Assertion : Noble gases are inert in nature.
Reason : Noble gases have zero valency.
Answer:
(A) Both the assertion and the reason are correct and the reason is the correct explanation of the assertion.
Very Short Answer Type Questions
Question 1.
What is the law of constant proportions?
Answer:
The law of constant proportions states that “a pure chemical compound always consists of the same elements that are combined together in a fixed proportion by mass.”
Question 2.
Define atom.
Answer:
The smallest particle of matter, which can take part in a chemical reaction is called atom.
Question 3.
What is molar mass? What are its units?
Answer:
The mass of one mole of a substance, i.e., Avogadro’s number of particles (6.022 x 1023 particles) is called its mass. Its unit is gram per mole (g/mol) or kilogram per mole (kg/mol).
Question 4.
Define atomicity.
Answer:
Atomicity is defined as the number of atoms present in a molecule. For example, atomicity of NH3 is 4 because there is one atom of N and three atoms of H.
![]()
Question 5.
Calculate the formula unit mass of Na2CO3. (Atomic mass of Na = 23u, C = 12u, O = 16u).
Answer:
The formula unit mass of a substance is the sum of the atomic masses of all the atoms in the formula unit of an ionic compound.
Therefore, formula unit mass of Na2CO3 = (2 × 23) + (1 × 12) + (3 × 16) = 46 + 12 + 48 = 106u.
Question 6.
Write the definition of a cation and an anion.
Answer:
- Cation: It is the positively charged ion, e.g., Na+ which is attracted towards cathode in an electric field.
- Anion: It is the negatively charged ion, e.g., Cl– which is attracted towards anode in an electric field.
Question 7.
Define law of conservation of mass.
Answer:
It states that “mass can neither be created nor destroyed in a chemical reaction”.
Question 8.
‘Atoms of most elements are not able to exist independently. Name two atoms which exist as independent atoms.
Answer:
Noble gases such as argon (Ar) and helium (He) exist independently as atoms.
Question 9.
Write the atomicity of the following:
(a) Sulphur
(b) Phosphorus
Answer:
(a) Polyatomic (Octa – atomic)
(b) Tetra – atomic
Question 10.
State the number of hydrogen atoms in 1g of hydrogen.
Answer:
One gram of hydrogen = One mole = 6.022 × 1023 atoms.
Analysing & Evaluating Questions
Question 11.
An element ‘Z’ forms the following compounds when it reacts with hydrogen, chlorine and oxygen.
ZH3, ZCl3 and Z2O3
(a) What is the valency of element ‘Z’?
(b) Is element ‘Z’ a metal or a non-metal?
Answer:
(a) The valency of ‘Z’ is 3.
(b) Element ‘Z’ is a metal because it is electropositive and is reacting with non – metals.
Question 12.
You are provided with a fine white coloured powder which is either sugar or salt. How would you identify it without tasting?
Answer:
This can be done by heating a little sample of each on a nickel spatula.
- If it gets charred, the sample is sugar.
- If it remains unaffected, it is salt.
Question 13.
An atom of element A is 1.92 times heavier that an atom of carbon – 12. What is the atomic mass of the element A?
Answer:
Atomic mass of A = 12.0g / mol × 1.92 = 23.04g/mol
Short Answer Type Questions
Question 1.
Write two drawbacks of Dalton’s atomic theory.
Answer:
Drawbacks of Dalton’s atomic theory:
- According to modem theory, atom is not the ultimate indivisible particle of matter. Today, we know that atoms are divisible, i.e., they are themselves made up of particles (protons, electrons, neutrons, etc.),
- In case of isotopes of an element, the assumption that atoms of the same element have same mass does not hold good.
Question 2.
What is meant by the term ‘molecule’? Give example.
Answer:
A molecule is the smallest particle of an element or a compound capable of independent existence under ordinary conditions. It shows all the properties of the substance, e.g., molecule of oxygen is O2, Ozone is O3, phosphorus is P4, sulphur is S8, etc.
Question 3.
Define one mole. Illustrate its relationship with Avogadro’s constant.
Answer:
One mole of any species (atoms, molecules, ions or particles) is that quantity in number, having a mass equal to its atomic or molecular mass in grams. The number of particles (atoms, molecules or ions) present in 1 mole of any substance is fixed with a value of 6.022 × 1023. This number is called Avogadro’s number.
Question 4.
What is formula unit mass? How is it different from molecular mass?
Answer:
The formula unit mass of a substance is a sum of the atomic masses of all the atoms in one formula unit of a compound. The constituent particles of formula unit mass are ions and the constituent particles of molecular mass are atoms.
Question 5.
Find the number of atoms in each of the following:
(a) 0.5moles of C atoms
(b) 2moles of N atoms
Answer:
(a) 0.5mole of C atoms : Number of atoms in 1 mole of C atoms = 6.022 × 1023 atoms
Number of atoms in 0.5 mole of C atoms = 6.022 × 1023 × 0.5 atoms = 3.011 × 1023 atoms.
(b) 2moles of N atoms : Number of atoms in 1 mole of N atoms = 6.022 × 1023 atoms Number of atoms in 2 moles of N atoms
= 6.022 × 1023 × 2
= 1.2044 × 1023 atoms.
![]()
Question 6.
What do you mean by symbols of elements?
Answer:
In order to write the chemical reactions conveniently, each element is represented by a ‘letter’ or a group of two letters called symbol. Thus, hydrogen is represented as ‘H’ and calcium is represented as ‘Ca’.
Question 7.
With the help of an example, state the significance of a symbol of an element.
Answer:
The symbol of oxygen is O. As an example, let us give the significance of the symbol O.
- O represents oxygen element.
- O represents one atom of oxygen element.
- O represents 16 atomic mass unit.
Question 8.
How do atoms exist?
Answer:
Individual atoms of most elements do not exist independently. These either aggregate to form elements or form molecules or ions which also aggregate in large numbers to form the matter that is available to us. Noble gases like helium, neon, etc., are an exception.
Question 9.
How does an atom differ from a molecule?
Answer:
An atom is the smallest particle of an element which may or may not have independent existence. On the other hand, molecule is the smallest particle of the element or compound which is capable of independent existence. For example, helium (H) is an atom and can exist as such, whereas hydrogen atom (H) cannot exist as such but exists as a molecule, i.e., Hexist as such, whereas hydrogen atom (H) cannot exist as such but exists as a molecule, i.e., H2. A molecule may be made up of similar atoms (homoatomic molecule such as O2) or dissimilar atoms (heteroatomic molecule such as HCl).
Question 10.
How many hydrogen and oxygen atoms are obtained when one molecule of water decomposes?
Answer:
It takes two molecules of the diatomic hydrogen gas to combine with one molecule of the diatomic oxygen gas to produce two molecules of water. In other words the ratio of hydrogen to oxygen is 2 : 1 the ratio of hydrogen to water is 1 : 1 and the ratio of oxygen to water is 1 : 2.
Question 11.
What are ionic compounds?
Answer:
Compounds containing charged species are known as ionic compounds. Such compounds are formed from combination of metals with non – metals. For example, sodium chloride is an ionic compound. Its constituent particles are positively charged sodium ions (Na+) and negatively charged chloride ions (CF–)
Question 12.
Name the ions in the following compounds. Sodium fluoride, potassium bromide, calcium oxide, silver sulphide, magnesium oxide
Answer:
(a) Sodium fluoride : Sodium cation and fluoride anion
(b) Potassium bromide : Potassium cation and bromide anion
(c) Calcium oxide : Calcium cation and oxide anion
(d) Silver sulphide : Silver cation and sulphide anion
(e) Magnesium oxide : Magnesium cation and oxygen anion
Question 13.
What is valency? What is its use?
Answer:
The valency of an element is the number of electrons an atom of the element uses to combine with atoms of other elements – it is the combining power of an atom of the element. In an atom, the valence electrons are the electrons that can be used in combining with other atoms. These are the electrons in the orbitals of the outermost shell (also called valency shell). It is not in all cases that the valency of an atom equals the total number of its valence electrons. For example, oxygen has 6 valence electrons, but its valency is 2. Some elements may have more than one combining power (or valency), while others have just one.
Question 14.
Do all elements have a charged valency? Explain.
Answer:
All elements do not form ions so their valencies do not have a charge. For example, carbon and silicon have a valency of 4 and nitrogen has a valency of 3. Further, molecules containing only non – metals are formed without a charged valency. For example, in carbon tetrachloride (CCl4), carbon has a valency of 4 and chlorine has a valency of 1.
Question 15.
Write the symbol and valency of the following polyatomic ions. Ammonium, hydroxide, nitrate carbonate, sulphate, sulphite bicarbonate and phosphate. A molecule may be made up of similar atoms (homoatomic molecule such as O2) or dissimilar atoms (heteroatomic molecule such as HCl).
Answer:
| Polyatomic ion | Symbol | Valency |
| Ammonium | (NH4)+ | +1 |
| Hydroxide | (OH)– | -1 |
| Nitrate | (NO3)– | -1 |
| Carbonate | (CO3)2- | -2 |
| Sulphate | (SO4)2- | -2 |
| Sulphite | (SO3)2- | -2 |
| Bicarbonate | (HCO3)– | -1 |
| Phosphate | (PO4)3- | -3 |
Analysing & Evaluating Questions
Question 16.
In photosynthesis, 6 molecules of carbon dioxide combine with an equal number of water molecules through a complex series of reactions to give a molecule of glucose having a molecular formula C6H12 O6. How many grams of water would be required to produce 18g of glucose? Compute the volume of water so consumed assuming the density of water to 1g cm 3.
Answer:
The photosynthesis can be described by the reaction.
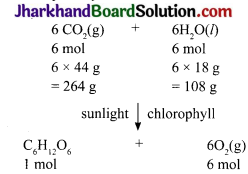
(6 × 12) + (12 × 1) + (6 × 16)g = (72 + 12 + 96)g = 180g
180g of glucose requires 108g water. 18g of glucose will require \(\frac{108 \mathrm{~g}}{180 \mathrm{~g}} \) x 8g = 10.8g
Then volume of water used
=\(\frac{\text { Mass }}{\text { Density }}=\frac{10.8}{1 \mathrm{~g} \mathrm{~cm}^{-3}}\)
= 10.8 cm3.
![]()
Question 17.
A compound was found to contain: Carbon = 55%, Oxygen = 36% and Hydrogen = 9%. What is the simplest whole number atomic ratio in the compound? The given data are processed as follows: Let us assume that the mass of the given compound is 100 gram.
Answer:
Carbon = 55% = \(\frac{55}{100}\) = 55 gram
Oxygen = 36% = \(\frac{36}{100}\) = 36 gram
Hydrogen = 9% \(\frac{9}{100}\) = 9 gram
\(\frac{55}{12}\) = 4.6 12
\(\frac{36}{16}\) 36/16 = 2.25 16
\(\frac{9}{1}\) 9/1 = 9.
Simplest atomic ratio
\(\frac{4.6}{2.25}\) = 2
\(\frac{2. 25}{2.25}\) = 1
\(\frac{9}{2.25}\) = 4
Thus, the simplest atomic ratio for the given compound is C : O : H = 2 : 1 : 4.
Long Answer Type Questions
Question 1.
What are the rules for writing the symbol of an element?
Answer:
IUPAC, i.e., International Union of Pure and Applied Chemistry approves names of elements. Symbols are the first one or two letters of the element’s name. The first letter of a symbol is always written as a capital letter (upper case) and second letter as a small letter (lower case).
For example:
Hydrogen → H
Helium → He
Some symbols are taken from the names of elements in Latin, German or Greek. For example,
- Symbol of iron is Fe, its Latin name is Ferrum.
- Symbol of sodium is Na, its Latin name is Natrium.
Question 2.
Describe the postulates of Dalton’s atomic theory.
Answer:
- Every element is composed of extremely small particles called atoms.
- Atoms of a given element are identical, both in mass and properties. Different chemical elements have different kinds of atoms. In particular, their atoms have different masses.
- Atoms can neither be created nor be destroyed or transformed into atoms of other elements.
- Compounds are formed when atoms of different elements combine with each other in the ratio of small whole numbers.
- The relative number and kinds of atoms in a given compound are constant.
Question 3.
Explain relative atomic mass and relative molecular mass.
Answer:
The atomic mass of an element is the relative mass of its atom as compared with the mass of a particular atom of carbon – 12 which is taken as 12 units. Thus, the atomic mass of an element indicates the number of times one atom of element is heavier than \(\frac{1}{2}\) of a carbon – 12 atom or (C – 12). For example, the atomic mass of oxygen is 16 which indicates that an atom of oxygen is 16 times heavier than \(\frac{1}{2}\) th mass of a carbon – 12 atom.
One atomic mass unit = \(\frac{1}{2}\) the mass of C – 12 atom.
Relative molecular mass is defined as the number of times one molecule of a substance or given element is heavier than \(\frac{1}{2}\) th of the mass of one atom of carbon – 12. For example, the relative molecular mass of water is 18. This means that the average mass of one molecule of water is 18 times the mass of one – twelfth of a carbon – 12 atom.
Question 4.
What is the utility of the mole concept?
Answer:
The utility of mole concept:
- From the number of moles of a substance, we can calculate the number of elementary particles
because the number of moles of a substance is directly proportional to the number of elementary particles. - One mole of a gas occupies 22. 4 litres at S. T. P (273 K and 1 atm).
- One mole of any gas particles in the same conditions of temperature and pressure occupies the same volume.
- One mole is equal to molecular mass in grams which is equal to 6.022 x 1023 atoms, molecules, ions, etc. Thus, we can calculate absolute masses of atoms and molecules.
Question 5.
(a) Calculate the mass of 0.72g molecules of CO2.
(b) Calculate the number of moles of iron in iron sheet containing 1022 atoms of iron.
Answer:
(a) Mass of one mole or 1 g molecules of CO2 = 12 + 32 = 44g
Mass of 0.72 g molecules of CO2 = 44 × 0. 72 = 31.68g.
(b) Number of moles containing 6.022 × 1023 atoms = 1 mole
Number of moles containing 1022 atoms of iron = \(\frac{1}{6.022 \times 10^{23}}\) × 1022 = 0.0166 mole
Moles of iron in iron sheet = 0. 0166 mole.
Question 6.
Define the term gram atom. How is it related to the mole and Avogadro’s number?
Answer:
The atomic mass of an element expressed in grams is called gram atomic mass or gram atom. One gram atom of any element contains 6.022 × 1023 (Avogadro’s number) atoms of the element. It is equal to one mole of atoms.
One gram atomic mass = 6.022 × 1023 atoms (Avogadro’s number) = 1 mole. The gram atomic mass of an element is equal to the mass of its = 6.022 × 1023 atoms.
For example, one gram atom of hydrogen atom weighs one gram and contains 6.022 × 1023 hydrogen atoms. One mole of hydrogen atom also weighs one gram. In other words, one atomic unit hydrogen means only one atom of hydrogen whereas one gram hydrogen has one mole atoms or 6.022 × 1023 atoms of hydrogen. Similarly, 16 u oxygen means only one atom of oxygen whereas 16 g oxygen has 6.22 × 1023 atoms of oxygen.
Question 7.
Calculate following:
(a) The mass of one atom of oxygen
(b) The mass of one molecule of oxygen
(c) The mass of one mole of oxygen gas
(d) The mass of one ion of oxygen
(e) The number of atoms in 1 mole of oxygen molecules
Answer:
(a) Mass of one atom of oxygen:
1 mole oxygen atom = 16g = 6.022 × 1023 atoms.
Mass of one atom of oxygen = 16/6.022 × 1023 =2.656 × 10-23g
(b) Mass of one molecule of oxygen:
1 molecule of oxygen = O2 = 2 × 16 = 32u
(c) Mass of one mole of oxygen gas:
1 mole of oxygen gas is O2 = 32g
(d) Mass of one ion of oxygen:
1 mole of oxygen = 6. 022 × 1023 atoms = 16g
Mass of one ion of oxygen =16/6.22 × 1023 = 2.65 × 10-23g
(e) Number of atoms in one mole of oxygen molecules:
1 mole of oxygen molecules, i. e.,
O2 = 6. 022 × 1023 molecules,
1 molecule of O2 = 2 atoms,
Number of atoms in 1 mole of oxygen molecule
= 6. 022 × 1023 × 2 atoms = 1.2044 × 1024 atoms.
Analysing & Evaluating Questions
Question 8.
Take 25 mL of 5% solution of lead nitrate in a beaker (100 mL). Take about 10 mL of dilute HCl in another beaker (100 mL). Weigh both the beakers together with their contents. Transfer dilute HCl to the lead nitrate solution and mix the solution. Allow the reaction to take place. Weigh both the beakers (one with the reaction mixture and the other empty) together.
1. What happens when HCl is added into the solution of lead nitrate?
2. Write the balanced chemical equation for the reaction that takes place.
3. What happens to the mass of the setup after the reaction?
4. Which law of chemical combination is illustrated by this activity?
Answer:
Weigh both the beakers (one with the reaction mixture and the other empty) together:
- When HCl is added to the solution of lead nitrate, white precipitate of lead chloride is formed.
- The white precipitate settles down.
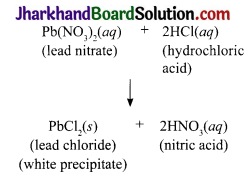
- There is no change in the mass of the setup after the reaction.
- The activity supports / illustrates the law of conservation of mass.
Activity
Take one of the following pairs of chemicals X and Y:
| X | Y |
| (a) Copper sulphate | Sodium carbonate |
| (b) Barium chloride | Sodium sulphate |
| (c) Lead nitrate | Sodium chloride |

Prepare separately a 5% solution of any one pair of substances listed under X and Y each in water:
- Take a little amount of solution of Y in a conical flask and some solution of X in an ignition tube.
Hang the ignition tube in the flask carefully such that the solutions do not get mixed. Put a cork on the month of the flask as shown in the figure. - Weigh the flask with its contents carefully.
- Now tilt and swirl the flask, so that the solutions X and Y get mixed.
- Weigh again.
- What happens in the flask?
- Do you think that a chemical reaction has taken place?
- Why should we put a cork on the mouth of the flask?
- Does the mass of the flask and its contents change?
Observations
- Some precipitate is formed in all cases.
- A chemical reaction takes place in all the cases.
- Cork is put on the mouth of the flask so that no material escapes out or splits out on swirling.
- The mass of the flask and its contents are the same before the reaction and after the reaction.
Value Based Questions
Question 1.
The idea of divisibility of matter was considered long back in India around 500 B, C. An Indian philosopher Maharishi Kanad, postulated that if we go on dividing matter, we shall end up getting smaller and smaller particles. Ultimately, a time will come when we shall come across the smallest particles, beyond which further division will not be possible. Another Indian philosopher Pakudha Katayama, eleborated his doctrine and said that these particles normally exist in a combined form and give us various forms of matter which find diverse use in our daily life.
1. Later on what name was given to these indivisible particles by Greek philosopher, Democritus.
2. What conclusion can you draw from the above paragraph in the social context?
Answer:
- Atoms.
- United we stand, divided we fall. Being together we can hope to achieve great heights.
![]()
Question 2.
Lavoisier, along with other scientists, observed that many compounds were formed from two or more elements. All these compounds had the same elements in the same proportions, irrespective of the source of compounds. For example, in a compound of water taken from sea, well or rain, hydrogen and oxygen are present in the ratio 1 : 8 by mass. Similarly, in ammonia, nitrogen and hydrogen are always present in the ratio 14 : 3 by mass, whatever be its source.
1. What name was given to this behaviour of chemical compounds? How is it formulated?
2. Does such properties of a compound related to human behaviour?
Answer:
- Above behaviour led to the law of definite proportions. This law states that in a chemical substance, the elements are always present in definite proportions by mass,
- Human behaviour is quite complicated. To a good extent, it also depends on the environment from where a person comes.