JAC Board Class 9th Science Important Questions Chapter 4 Structure of the Atom
Multiple Choice Questions
Question 1.
Almost the entire mass of an atom is concentrated in the?
(a) proton
(b) electron
(c) nucleus
(d) neutrons
Answer:
(c) nucleus
Question 2.
Positive charge is carried by:
(a) X – rays
(b) cathode rays
(c) Y – rays
(d) anode rays
Answer:
(d) anode rays
Question 3.
Which one is not true for isotopes?
(a) Similar mass number
(b) Similar chemical properties
(c) Similar atomic number
(d) Similar electronic configuration
Answer:
(a) Similar mass number.
Question 4.
Electron was discovered by
(a) Chadwick
(b) Thomson
(c) Goldstein
(d) Bohr
Answer:
(b) Thomson.
![]()
Question 5.
The size of an atom is decided by
(a) mass of the atom
(b) number of protons
(c) number of protons and neutrons
(d) number of electrons
Answer:
(d) number of electrons.
Question 6.
Calcium has 20 electrons. These occupy K, L, M and N shells. Which shell or shells are incomplete?
(a) L, M, N shells
(b) M, N shells
(c) N shell
(d) K, M, L, N shells
Answer:
(c) N shell
Question 7.
The K, L and M shells of an atom are full. Its atomic number is:
(a) 18
(b) 20
(c) 10
(d) 12
Answer:
(a) 18.
Question 8.
Cathode rays are deflected towards?
(a) positive electrode
(b) negative electrode
(c) both electrodes
(d) none of the electrode
Answer:
(a) positive electrode.
Question 9.
The absolute charge of an electron is?
(a) -1.6 × 10-19C
(b) +1.6 × 10-19C
(c) 0.16 × 10-19 C
(d) 16 × 10-19 C
Answer:
(a) -1.6 × 10-19C.
Question 10.
The proton is heavier than an electron by?
(a) 1850 times
(b) 1840 times
(c) 1000 times
(d) 100 times
Answer:
(b) 1840 times.
Question 11.
Carbon – 12 atom has:
(a) 6 electrons, 6 protons, 6 neutrons
(b) 6 electrons, 12 protons, 6 neutrons
(c) 12 electrons, 6 protons, 6 neutrons
(d) 18 electrons, 6 protons and 6 neutrons
Answer:
(a) 6 electrons, 6 protons, 6 neutrons.
Question 12.
Chadwick got the Nobel prize for the discovery of?
(a) protons
(b) neutrons
(c) electrons
(d) none of these
Answer:
(b) neutrons.
Question 13.
The volume of the nucleus of an atom when compared to the extranuclear part is?
(a) bigger
(b) smaller
(c) same size
(d) unpredictable
Answer:
(b) smaller.
Question 14.
The isobars among the following are
(a) \({ }_{20}^{40} \mathrm{Ca}\), \({ }_{17}^{34} \mathrm{Cl}\)
(b) \({ }_{18}^{40} \mathrm{Ar},{ }_{19}^{40} \mathrm{Ca}\)
(c) \({ }_{8}^{16} \mathrm{O},{ }_{8}^{18} \mathrm{O}\)
(d) \({ }_{7}^{19} \mathrm{X},{ }_{7}^{13} \mathrm{Y}\)
Answer:
(b) \({ }_{18}^{40} \mathrm{Ar},{ }_{19}^{40} \mathrm{Ca}\).
Question 15.
Outermost shell of an atom cannot accommodate more electrons than
(a) 2
(b) 8
(c) 18
(d) 16
Answer:
(b) 8.
Analysing & Evaluating Questions
Question 16.
The number of electrons in an element X is 15 and the number of neutrons is 16. Which of the following is correct representation of the element?
(a) \({ }_{15}^{31} X\)
(b) \({ }_{31}^{16} X\)
(c) \({ }_{16}^{15} X\)
(d) \({ }_{15}^{17} X\)
Answer:
(a) \({ }_{15}^{31} X\).
![]()
Question 17.
The ion of an element has 3 positive charges. Mass number of the atom is 27 and the number of neutrons is 14. What is the number of electrons in the ion?
(a) 13
(b) 10
(c) 14
(d) 16
Answer:
(b) 10.
Question 18.
Which of the following in figures given below do not represent Bohr’s model of an atom correctly?
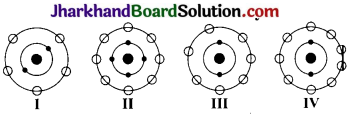
(a) I and II
(b) II and III
(c) II and IV
(d) I and IV
Answer:
(c) II and IV.
Assertion Reason Questions
Directions: In the following questions, the Assertions and the Reasons have been put forward Read the statements carefully and choose the correct alternative from the following:
(A) Both the assertion and the reason are correct and the reason is the correct explanation of the assertion.
(B) The assertion and the reason are correct but the reason is not the correct explanation of the assertion.
(C) The assertion is true but the reason is false.
(D) Both the statements are false.
1. Assertion : Nucleus of an atom is positively charged.
Reason : Neutrons lie in the nucleus of an atom.
Answer:
(B) The assertion and the reason are correct but the reason is not the correct explanation of the assertion.
2. Assertion : Electrons revolving in the orbit never fall in the nucleus.
Reason : Opposite charges of nucleus and electrons maintain centripetal force.
Answer:
(C) The assertion is true but the reason is false.
3. Assertion : Valency of aluminum is 3.
Reason : Valency is the number of electrons present in the outermost shell.
Answer:
(C) The assertion is true but the reason is false.
4. Assertion : Mass of an atom lies in its nucleus.
Reason : Nucleus of an atom is composed of neutrons and protons.
Answer:
(A) Both the assertion and the reason are correct and the reason is the correct explanation of the assertion.
5. Assertion : Hydrogen and deuterium have similar chemical properties.
Reason : Hydrogen and deuterium have the same electronic configuration.
Answer:
(A) Both the assertion and the reason are correct and the reason is the correct explanation of the assertion.
Very Short Answer Type Questions
Question 1.
What is an electron? What are its relative mass and charge?
Answer:
An electron is that sub – atomic particle which is negatively charged and has a mass of about 1/1840 times that of an atom of hydrogen.
Question 2.
What are nucleons?
Answer:
The sub – atomic particles (protons and neutrons) present in the nucleus of an atom are known as nucleons.
![]()
Question 3.
Why Bohr’s orbits are called stationary states?
Answer:
According to Bohr’s theory, electrons revolve around the nucleus and they have a fixed amount of energy. Thus, they are called stationary states.
Question 4.
What is an orbit?
Answer:
Orbit is the path around the nucleus in which the electron revolves.
Question 5.
What is meant by the electronic configuration of elements?
Answer:
The systematic arrangement of electrons in different orbits or shells of an atom of an element is known as the electronic configuration of that element.
Question 6.
Why did Rutherford select a gold foil in his α – ray scattering experiment?
Answer:
It is because gold has high malleability and it can be hammered into thin sheets.
Question 7.
Will Cl – 35 and Cl – 37 have different valencies?
Answer:
No. It is because these are isotopes of chlorine that have same atomic number but different mass numbers.
Question 8.
Calculate the number of neutrons present in the nucleus of an element X which is represented as X 15.
Answer:
\({ }^{31} \mathrm{X}_{15}\)indicate that no. of protons = 15 and mass number = 31
Mass number = No. of protons + No. of neutrons = 31
Number of neutrons = 31 – number of protons = 31 – 15 = 16
Question 9.
Why do Helium, Neon and Argon have zero valency?
Answer:
Helium, Neon and Argon have 2, 8 and 8 electrons respectively in their outermost shell, so there is no need for them to gain or lose electrons. Hence, they have zero valency.
Question 10.
Name two elements each with same number of protons and neutrons?
Answer:
Carbon (protons = neutrons = 6) and oxygen (protons = neutrons = 8)
Question 11.
Can the value of Z be same for two different atoms? (Z = atomic number)
Answer:
No, two different atoms cannot have same atomic number.
Question 12.
Name the scientist who discovered protons and neutrons in an atom.
Answer:
Protons were discovered by E. Goldstein in 1866 and neutrons were discovered by J. Chadwick in 1932.
Question 13.
What is an octet? Why do the atoms want to complete their octet?
Answer:
When the outermost shell of an atom is completely filled, it is said to be an octet. Atoms want to complete their octet because they want to become stable.
![]()
Question 14.
Find the valency of \({ }_{7}^{14} \mathrm{~N}\) and \({ }_{17}^{35} \mathrm{Cl}\)
The atomic number of nitrogen = 7,
number of protons = 7,
number of electrons = 7
Electronic configuration = K L M
= 2, 5,
⇒ Valency = 3
It will either gain three electrons or share 3 electrons to complete its octet.
The atomic number of chlorine = 17,
number of protons = 17,
number of electrons = 17
Electronic configuration = K L M
= 2, 8, 7 = Valency = 1 It will gain 1 electron to complete its octet.
Question 15.
Give the difference between three sub-atomic particles.
Answer:
Three sub – atomic particles are electrons, proton and neutron:
| Particle | Electron | Proton | Neutron |
| Discovered by | J.J Thomson | E. Goldstein | J. Chadwick |
| Charge | -1 | +1 | 0 |
| Symbol | e | P | n |
| Mass | 1/1840u | 1u | 1u |
Analysing & Evaluating Questions
Question 16.
In a sample of ethyl ethanoate (CH3COOC2H5), the two oxygen atoms have the same number of electrons but different number of neutrons. What can be the possible reason for it?
Answer:
The reason for it is that the two oxygen atoms are isotopes.
Question 17.
In response to a question, a student stated that in an atom, the number of protons is greater than the number of neutrons, which in turn is greater than the number of electrons. Do you agree with the statement? Justify your answer.
Answer:
No. The statement is wrong In a neutral atom, the number of protons and electrons are always equal.
Question 18.
In the Gold foil experiment of Geiger and Marsden, that paved the way for Rutherford’s model of an atom, about 1.00% of the α – particles were found to deflect at angles > 50°. If one mole of α – particles were bombarded on the gold foil, compute the number of α – particles that would deflect at angles less than 50°.
Answer:
No. of particles deflected by < 50°
=\(\frac{99}{100} × \) × 6.022 x 1023 = 5.96 × 1023.
Short Answer Type Questions
Question 1.
In what way did Thomson propose the atomic model?
Answer:
Thomson proposed the model of an atom to be similar to a Christmas pudding The electrons are studded like currents in a positively charged sphere like Christmas pudding and the mass of the atom is supposed to be uniformly distributed.
Question 2.
What is an electron? State its relative mass and charge.
Answer:
An electron is a negatively charged particle found in the atoms of all the elements. Its relative mass is 1/1840
(a) m.u. and relative charge is negative.
Question 3.
What is a discharge tube?
Answer:
A discharge tube is a glass tube about 70 cm long and having a diameter of 5 cm. Two metal electrodes are sealed at the two ends, one of which is connected to the negative terminal of a battery and the other to the positive terminal. A side tube is fused at the centre of the glass tube which serves to pump out air from it, using a suction pump.
Question 4.
What are anode rays? State three properties of anode rays.
Answer:
Anode rays are stream of positively charged particles shot out from the anode of a discharge tube when a current is passed through a gas.
- Anode rays travel in straight lines. They cast shadows of the objects placed in their path.
- Anode rays can produce mechanical effects. This is evident by the fact that they can rotate a light paddle.
- Anode rays are positively charged as they are deflected towards the negative plate in an electric field.
Question 5.
What happens to the e/m ratio of positive rays and why?
Answer:
The mass and charge of positively charged particles depend upon the gas, which is taken in the discharge tube. Different gases contain particles having different masses and different charges and consequently give different types of positive rays. In other words, the charge – to – mass ratio (e/m) is not constant.
Question 6.
A student weighs 30 kg Suppose his entire body is made up of electrons. How many electrons are there in his body? Compare the total number of electrons in his body with the population of India.
Answer:
Mass of electron = 9.1 × 10-31kg.
Number of electrons in the body of student = Total mass/Mass of each electron = 30 kg/ 9.1 × 10-31 kg Therefore, the student is made up of approximately 3 . 29 × 1031 electrons. The population of India is about 130 crores.
\(\frac{3.29 \times 10^{31}}{1.3 \times 10^{9}}\)
= 2.53 × 1022
Therefore, number of electrons in the body of the student is 2.53 × 1022 times the population of India.
Question 7.
When an electron jumps from energy level K to energy level L, why is the energy of the atom increased?
Answer:
When an electron revolves around its nucleus in its orbit, sometimes it gain energy and it jumps from lower energy shell to higher energy shell. The energy of electrons increases hence, the energy of atom also increases.
![]()
Question 8.
State the similarities and dissimilarities between protons and neutrons.
Answer:
1. Similarities:
- Both protons and neutrons are present in the nucleus of an atom.
- The mass of a neutron is approximately equal to the mass of a proton, i.e., 1.67 × 10-27 kg.
2. Dissimilarities:
- Protons possess a unit positive charge (1.6 × 10-19coulomb).
- Neutron carries no charge, i.e., it is an electrically neutral particle.
Question 9.
Argon atom has 18 electrons. How many energy shells or orbits are incomplete?
Answer:
Distribution of 18 electrons in argon atom is K – shell = 2 electrons, L – shell = 8 electrons and M – shell = 8 electrons. K – shell and L – shell have maximum capacity of 2 and 8 electrons respectively. M – shell has a capacity of 18 electrons. Since, it is the outermost orbit and there is a rule that ‘the maximum number of electrons that can be accommodated in the outermost orbit is 8. In the present case, M – shell is also complete. Thus, in argon atom, no shell is incomplete. Argon atom has three comletely filled energy shells.
Question 10.
An element has 16 protons. How many electrons will be present in K, L and M shells of its atom? What will be its electrovalency?
Answer:
No. of electrons = no. of protons = 16
Electronic configuration: K = 2, L= 8, M = 6.
Valency or electrovalency is 8 – 6 = 2.
Question 11.
Define the term isotope. State the similar properties of isotopes.
Answer:
Atoms of the elements having the same atomic number but different atomic masses are called isotopes. This means isotopes of an element have the same number of protons but different number of neutrons in their nuclei. For example,
1. Carbon has two isotopes:
- \({ }_{6}^{12} \mathrm{C}\) – Atomic number 6 and mass number 12 and thus has 6 neutrons.
- \({ }_{6}^{14} \mathrm{C}\) – Atomic number 6 and mass number 14 and thus has 8 neutrons.
Properties of isotopes are:
- Isotopes of an element have same atomic number.
- Isotopes of an element have similar chemical properties.
- Isotopes of an element have similar number of electrons.
- Isotopes of an element have similar electronic configurations.
Question 12.
Why is carbon – 14 isotope radioactive whereas carbon – 12 is not?
Answer:
C – 12 atom has 6 protons and 6 neutrons, so it is not radioactive. C – 14 atom has 6 protons but 8 neutrons and thus has more neutrons than protons in its nucleus, therefore, its nucleus becomes unstable and shows radioactivity.
Analysing & Evaluating Questions
Question 13.
An atom ‘M’ of an element reacts with oxygen to form M203. Calculate the valency of the element ’M’.
Answer:
Two atoms of element ‘M’ combine with 3 atoms of oxygen.
∴ Number of oxygen atoms combining with one atom of element ‘M’ =\(=\frac{3}{2}\)
Therefore, the valency of element ‘M’ = \(=\frac{3}{2}\) × 2 = 3.
Question 14.
When high electrical potential is applied across a gas at very low pressure (≈10-5 s atm), the walls of the discharge tube opposite to the cathode start glowing with a faint greenish light. What is the cause behind this observation?
Answer:
Fluorescence of the glass walls due to bombardment by the rays emitted from the cathode is responsible for faint greenish light.
Long Answer Type Questions
Question 1.
What are cathode rays? How are they formed?
Answer:
Cathode rays are a stream of negatively charged particles. These particles, called electrons, are shot from the metal cathode of a discharge tube when an electric current is passed through a gas at a very low pressure. A discharge tube is a long glass tube having two metal electrodes. When the pressure of air in the discharge tube is reduced to 0.001 mm of mercury and a high voltage is applied to the electrodes, the emission of light by air stops. But it is noticed that the wall of the discharge tube at the end opposite to the cathode begins to glow with greenish light. Since these rays originate at the cathode, they are known as cathode rays.
Question 2.
Who discovered the nucleus within an atom? How?
Answer:
Ernest Rutherford discovered the nucleus within an atom in his alpha – ray scattering experiment. The arrangement of the alpha – particle scattering experiment is as follows:
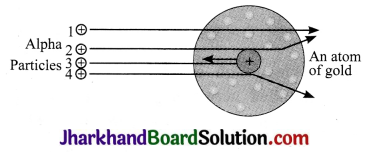
Rutherford produced a narrow beam of particles from a radioactive source (e.g., radium or polonium), which was allowed to strike an extremely thin gold foil. Rutherford proposed that if the spherical model proposed earlier, which made a uniform distribution of positive and negative particles was correct, then the alpha particle striking the gold atoms would be uniformly deflected However the observations were:
- Most of the alpha particles passed straight through the gold foil without suffering any deflection from their original path
- A few of them were deflected through small angles, while a very few deflected to a large extent.
- A very small percentage (1 in 12,000) was deflected through 180° (turned back).
This showed that positive mass is concentrated only at the centre of the atom. This was identified as nucleus.
Question 3.
Describe Rutherford’s model of an atom.
Answer:
Rutherford’s model of an atom:
- The atom of an element consists of a small positively charged nucleus which is situated at the centre of the atom and which carries almost the entire mass of the atom.
- The electrons are distributed in the empty space of the atom and are revolving around the nucleus at high speed.
- The number of electrons in an orbit is equal to the number of positive charges (protons) in the nucleus. Hence, the atom is electrically neutral.
- The volume of the nucleus is negligibly small as compared to the volume of the atom.
- Most of the space in the atom is empty.
- The arrangement is just like the solar system.
Question 4.
What are the important properties of cathode rays?
Answer:
The important properties of cathode rays:
- Cathode rays travel in straight lines. That is why, cathode rays cast shadow of any solid object placed in their path.
- Cathode rays set a paddle wheel into motion when it is placed in the path of these rays on the bladder of the paddle wheel. Hence, they are made up of material particles.
- Cathode rays consist of negatively charged particles. When cathode rays are subjected to an electrical field, these get deflected towards the positively charged plate (anode).
- Cathode rays heat the object only on which they fall. The cathode ray particles possess kinetic energy. When these particles strike an object, a part of the kinetic energy is transferred to the object. This causes a rise in the temperature of the object.
- Cathode rays cause green fluorescence on glass surface, i.e., the glass surface on which the cathode rays strike shows a coloured shine.
- Cathode rays can penetrate through thin metallic sheets.
- Cathode rays ionise the gases through which they travel.
- When cathode rays fall on certain metals, such as copper, X – rays are produced The X – rays are not deflected by electrical or magnetic fields. X – rays pass through opaque materials such as black paper, but stopped by solid objects such as bones.
- Cathode rays travel with speed nearly equal to that of light.
![]()
Question 5.
State the postulates of Bohr’s model of an atom. Draw a diagram of Bohr’s model of an atom.
Answer:
The postulates of Bohr’s model of atom are as follows:
(a) In an atom, the electrons revolve around the nucleus in certain definite circular paths called orbits or shells. These are represented by the letters K, L, M, N… or the numbers n = 1,2, 3. 4…..
(b) The maximum number of electrons present in a shell is given by the formula 2n2, where ‘n’ is the orbit number or energy level index, 1,2,3……. Hence, the maximum number of electrons in different shells is as follows, First orbit (K shell) will be = 2 × 12 = 2, second orbit (or L shell) will be = 2 × 22 = 8, third orbit (M shell) will be = 2 × 32 = 18 and so on.
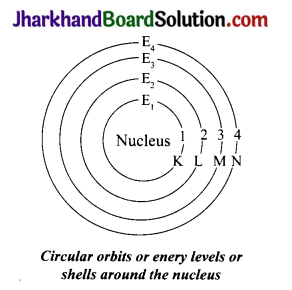
(c) The maximum number of electrons that can be accommodated in the outermost orbit is 8.
(d) Electrons are not accommodated in a given shell, unless the inner shells are filled That is, the shells are filled in a step – wise manner.
(e) While revolving in discrete orbits, the electrons do not radiate energy.
Question 6.
Atom A has a mass number of 238 and atomic number of 92 and atom B has mass number 235 and atomic number 92.
1. How many protons do atoms A and B have?
2. How many neutrons do atoms A and B have?
3. Are atoms A and B isotopes of the same element? How?
Answer:
- Both atoms A and B have the same number of protons = atomic number = 92
- Number of neutrons in atom A = Mass number – atomic number = 238 – 92 = 146 Number of neutrons in atom B = 235 – 92 = 143
- Atoms A and B are isotopes of the same element as these have similar chemical properties. This is because both have same number of electrons.
Analysing & Evaluating Questions
Question 7.
The radiation emitted by the cathode in a discharge tube at very low pressure (≈ 10 – 5 atm) is found to get deflected towards positive field, can set a paddle wheel into motion, cast shadow of any solid object placed in its path and travel with the speed of light.
(a) Identify the radiation.
(b) Mention its properties which are responsible for the observed phenomena.
Answer:
- The radiation is cathode rays.
- The properties of the emitted radiation which are responsible for the observed phenomena are:
- get deflected towards positive field indicates that it consists of negatively charged particles.
- can set a paddle into motion indicates that the cathode rays consist of material particles.
- casts shadow of any solid when placed in its path indicates that cathode rays travel in straight line.
- travel with the speed of light which indicates that particles in it are associated with electrical and magnetic fields, that is, the rays are electromagnetic radiation.
Activity – 1
- Rub a comb on your dry hair.
- Does the comb then attract the small pieces of paper?
Now bring the comb close to some small pieces of paper.
Observation - On rubbing, some electrons will move from hair to comb Thus the comb has negative charge.
- The negatively charged comb will attract the paper pieces.
Activity – 2
- Rub a glass rod with a silk cloth and bring the rod near an inflated balloon.
- Observe what happens.
Observation
On rubbing, some electrons will move from glass rod to the silk cloth. Thus the glass rod becomes positively charged This charged glass rod now attracts the inflated balloon.
Value Based Questions
Question 1.
The distribution of electrons into different orbits of an atom was suggested by Bohr and Bury. The maximum number of electrons in a shell is given by 2n where n is the orbit number or energy level, i.e., K, L, M, N Further, shells are filled in a stepwise manner and that the outermost shell cannot accommodate more than 8 electrons. Shyam was asked to give the distribution of 20 electrons contained in an atom. He suggested the distribution as 2, 8, 10 as per formulation (2n2). The teacher – guided him and explained him the correct distribution.
1. What should be the correct distribution? Which shells are completely filled?
2. What moral values were given by the teacher to Shyam?
Answer:
- The correct distribution is 2, 8, 8, 2. The shells (K, L) are completely filled.
- The teacher told Shyam that a principle should be applied in totality to solve a problem.
Question 2.
00Isotops are the variants of a particular element. While all isotopes of a given element show the same number of protons, each isotope differs from the other in the number of neutrons. Due to particular proton – neutron ratio in the nucleus, some isotopes are stable and some are unstable. The latter are called radioactive elements. In the natural element, the amount of radioactivity is quite small and remains ineffective. As radio – elements find use in chemical reactions, medicines and as fuel and even face – wash, these are enriched for specific purpose.
1. Should the enrichment/isolation of radio – elements be banned or continued?
2. As a common man, show your concern to the production and use of isotopes?
Answer:
- The isolation/enrichment of isotopes should be continued as these are very useful.
- The common concern is that their exposure is dangerous to life and vegetation. These should be handled with care and stored properly.