JAC Board Class 9th Science Important Questions Chapter 2 Is Matter Around Us Pure
Multiple Choice Questions
Question 1.
Which of the following shows Tyndall effect?
(a) Starch solution
(b) Salt solution
(c) Sugar solution
(d) Copper sulphate solution
Answer:
(a) Starch solution
Question 2.
To obtain toned and double toned milk from full cream milk we can perform
(a) distillation
(b) filtration
(c) sedimentation
(d) centrifugation
Answer:
(d) centrifugation
![]()
Question 3.
Fog and clouds are examples of
(a) suspension
(b) emulsion
(c) aerosol
(d) colloid
Answer:
(c) aerosol
Question 4.
A mixture of ammonium chloride and sand can be separated by
(a) decantation
(b) sublimation
(c) centrifugation
(d) evaporation
Answer:
(b) sublimation
Question 5.
…………. may be termed as a pure substance.
(a) Soft drink
(b) Aerosol
(c) Sodium chloride
(d) Soil
Answer:
Question 6.
…………. is a chemical change.
(a) Evaporation
(b) Heating of copper and sulphur
(c) Freezing of water
(d) Mixing of H2 and 02
Answer:
(b) Heating of copper and sulphur
Question 7.
A colloidal solution is a
(a) heterogeneous and transparent mixture
(b) homogeneous mixture
(c) heterogeneous mixture in which particles can be seen with naked eyes
(d) heterogeneous mixture in which particles cannot be seen with naked eyes
Answer:
(d) heterogeneous mixture in which particles cannot be seen with naked eyes
Question 8.
Shaving cream is a colloidal solution of
(a) gas in liquid
(b) liquid in liquid
(c) solid in liquid
(d) gas in solid
Answer:
(a) gas in liquid
Question 9.
………… represents a physical change.
(a) Electric current passed through water
(b) Electric current is passed through a bulb and it glows
(c) Burning of a candle
(d) Making of curd from milk
Answer:
(b) Electric current is passed through a bulb and it glows
Question 10.
10 gram each of iron filings and sulphur podwer is taken in a china dish. Which of the following activities gives a compound?
(a) Separate iron filings with the help of a magnet
(b) Add carbon disulphide, stir well and filter
(c) Mix and crush the mixture
(d) Heat strongly till red hot while mixing
Answer:
(d) Heat strongly till red hot while mixing
Question 11.
Choose a colloid out of the following.
(a) Air
(b) NaCl in water
(c) Emulsion
(d) Alloy
Answer:
(c) Emulsion
Question 12.
Chalk powder dissolved in water is an example of
(a) suspension
(b) true solution
(c) colloid
(d) saturated solution
Answer:
(a) suspension
Analysing & Evaluating Questions
Question 13.
Two substances, A and B were made to react to form a third substance, A2B according to the following reaction: 2A + B → A2B Which of the following statements concerning this reaction are incorrect?
I. The product A2B shows the properties of substances A and B.
II. The product will always have a fixed composition.
III. The product so formed cannot be classified as a compound.
IV. The product so formed is an element.
(a) I, II and III
(b) II, III and IV
(c) I, III and IV
(d) I, II, and IV
Answer:
(c) I, III and IV
![]()
Question 14.
Two chemical species X and Y combine together to form a product P which contains both X and Y: X + Y → P X and Y cannot be broken down into simpler substances by simple chemical reactions. Which of the following statements concerning the species X, Y and P are correct?
I. P is a compound.
II. X and Y are compounds.
III. X and Y are elements.
IV. P has a fixed composition.
(a) I, II and III
(b) I, II and IV
(c) II, III and IV
(d) I, III, and IV
Answer:
(d) I, III, and IV
Question 15.
Arun has prepared 0.01% (by mass) solution of sodium chloride in water. Which of the following correctly represents the composition of the solutions?
(a) 1.00 g of NaCl + 100 g of water
(b) 0.11 g of NaCl + 100 g of water
(c) 0.01 g of NaCl+99.90 g of water
(d) 0.10 g of NaC 1 +99.90 g of water
Answer:
(c) 0.01 g of NaCl+99.90 g of water
Assertion Reason Questions
Directions: In the following questions, the Assertions and the Reasons have been put forward. Read the statements carefully and choose the correct alternative from the following:
(A) Both the assertion and the reason are correct and the reason is the correct explanation of the assertion.
(B) The assertion and the reason are correct but the reason is not the correct explanation of the assertion.
(C) The assertion is true but the reason is false.
(D) Both the statements are false.
1. Assertion: Colloids are heterogeneous mixtures.
Reason: Particles of colloids can scatter a beam of light.
Answer:
(B) The assertion and the reason are correct but the reason is not the correct explanation of the assertion.
2. Assertion: A solution contains solute dissolved in a solvent.
Reason: Solutions always exist in liquid state.
Answer:
(C) The assertion is true but the reason is false.
3. Assertion: Ethanol and water can be separated from their mixture by distillation process.
Reason: There is much difference between the boiling points of ethanol and water.
Answer:
(D) Both the statements are false.
4. Assertion: On heating, a saturated solution becomes unsaturated.
Reason: Solubility of a substance decreases with increasing temperature.
Answer:
(C) The assertion is true but the reason is false.
5. Assertion: Human blood is an impure substance.
Reason: The composition of human blood is variable.
Answer:
(A) Both the assertion and the reason are correct and the reason is the correct explanation of the assertion.
Very Short Answer Type Questions
Question 1.
Which of the following materials are pure substances? Butter, ghee, ink, water, banana, glass, sugar, blood, marble, wood Answer:Pure substances are sugar and water.
Question 2.
Define solution. If 10 mL of sulphuric acid is dissolved in 90 mL of H2O, calculate the concentration of sulphuric acid in the solution.
Answer:
A solution is a homogeneous mixture of two or more substances. Concentration of sulphuric acid in solution = \(\frac{10 \times 100}{100}\)
= 10% volume by volume.
Question 3.
Can we separate alcohol dissolved in water by using a separating funnel? Yes or No. Why?
Answer:
No, water and alcohol, when mixed, form a single layer being miscible and thus separating funnel cannot be used to separate alcohol dissolved in water.
Question 4.
What is the important criterion used in chromatography for separating the components of a mixture?
Answer:
Chromatography can be used for separating the components of a mixture if the components of the mixture exhibit different solubilities in the same solvent or a mixture of solvents.
Question 5.
Differentiate between the solvent and the solute.
Answer:
The component of the solution that dissolves the other component into it is called the solvent. The component of the solution that is dissolved in the solvent is called solute.
Question 6.
What is ‘tincture of iodine’?
Answer:
A solution of iodine in alcohol is known as tincture of iodine, ft has iodine (solid) as the solute and alcohol (liquid) as the solvent.
Question 7.
What is concentration of a solution?
Answer:
The amount of solute dissolved in the given amount of solution is called the concentration of that solution.
Question 8.
Why is water called a universal solvent?
Answer:
Water is called the “universal solvent” because it dissolves more substances than any other liquid.
![]()
Question 9.
Define Tyndall effect.
Answer:
Tyndall effect is the scattering of light as a light beam passes through a colloid.
Question 10.
Differentiate between emulsion and aerosol.
Answer:
When both the dispersed phase and the dispersing medium are liquid, the colloid is called an emulsion. For example, milk and face cream. When solid or liquid is dispersed in a gas, it is called aerosol. Example, smoke and fog.
Question 11.
How can you separate two liquids that have difference of boiling points less than 25K?
Answer:
To separate a mixture of two or more miscible liquids for which the difference in boiling points is less than 25K, fractional distillation is used.
Question 12.
What is crystallisation?
Answer:
Crystallisation is a process that separates a pure solid in the form of its crystals from a solution. The crystallisation method is used to purify solids. For example, the salt we get from sea water can have many impurities in it. To remove these impurities, the process of crystallisation is used.
Question 13.
Write any two applications of crystallisation.
Answer:
- Purification of salt that we get from sea – water.
- Separation of crystals of alum (phitkari) from impure samples.
Question 14.
What is an alloy?
Answer:
The homogeneous mixture of two or more metals or a metal and a non-metal is called an alloy. For example, steel is an alloy of iron and carbon.
Analysing & Evaluating Question
Question 15.
While diluting a solution of salt in water, a student by mistake added acetone (boiling point 56°C). What technique can be employed to get back the acetone? Justify your choice.
Answer:
Acetone can be obtained back by distillation and can be collected as distillate. This is because the boiling point of acetone (56°C) is much lower than that of water (100°C).
Question 16.
Sucrose (sugar) crystals obtained from sugarcane and beetroot are mixed together. Will it be a pure substance or a mixture? Give reasons for the same.
Answer:
It will be a pure substance because the chemical composition of sugar (sucrose) is always the same, whatever be its source.
Question 17.
Mixtures of three substances in water are filtered and the observations are shown in the figure given alongside. Which of the mixtures is:
(a) true solution?
(b) suspension?
(c) colloidal solution?
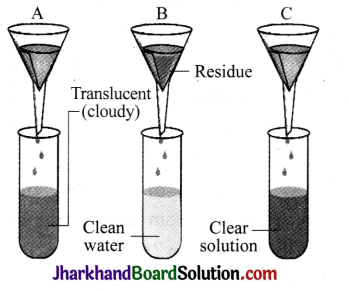
Answer:
(a) Mixture in C is a true solution.
(b) Mixture in B is a suspension.
(c) Mixture in A is a colloidal solution.
Short Answer Type Questions
Question 1.
Differentiate between mixtures and compounds.
Answer:
| Mixtures | Compounds |
| (a) Elements or compounds just mix together to form a mixture and no new substance is formed. | (a) Elements react to form new substances, i.e. compound |
| (b) A mixture has a variable composition. | (b) The composition of a compound is always fixed. |
| (c) A mixture shows the properties of its constituent substances. | (c) The properties of a compound are totally different from those of its constituents. |
| (d) The constituents can be separated fairly easily by physical methods. | (d) The constituents can be separated only by chemical or electrochemical reactions. |
Question 2.
Distinguish between a physical change and a chemical change.
Answer:
| Physical Change | Chemical Change |
| (a) No new substance is formed. | (a) New substance is formed. |
| (b) It is a reversible change. | (b) It is an irreversible change. |
| (c) The properties of constituents are retained. | (c) The properties of constituents are not retained. |
Question 3.
How will you separate a mixture of oil and water?
Answer:
To separate a mixture of oil and water, we need a separating funnel as both are immiscible liquids. Pour the mixtures in separating funnel and let the funnel stand undisturbed for a few minutes, so that separate layer of oil and water are formed. Open the stopcock of the separating funnel and pour out the lower layer of water carefully.
Question 4.
How will you establish that air is a mixture and not a pure substance?
Answer:
Air is a homogeneous mixture of several gases. The amounts of gases present in air at different places vary. No definite formula can be assigned to air. The different gases present in air are separable by a physical process, i.e., fractional distillation of liquid air. Hence, air is a mixture and not a pure substance.
Question 5.
Define the following terms:
(a) True solution
(b) Solute
(c) Solvent
(d) Solubility
Answer:
(a) True solution: A true solution is defined as a homogeneous mixture of two or more substances. It means that every portion of the solution has same properties, e.g., sugar dissolved in water, iodine in ethyl alcohol (tincture of iodine).
(b) Solute: The substance present in smaller proportion in a solution is called solute.
(c) Solvent: The substance present in larger proportion in a solution is called solvent. For example, if a homogeneous mixture or a solution is formed by dissolving 2 g salt in 100 g of water, then salt is the solute and water is the solvent.
(d) Solubility: The maximum amount of solid that can be dissolved in a given amount of the solvent at a particular temperature is termed a its solubility at that temperature.
Question 6.
State the difference between aqueous and non – aqueous solutions.
Answer:
True solutions obtained in water are aqueous solutions, e.g, vinegar. True solutions obtained without water, but in organic liquids, like alcohol, acetone, etc., are non-aqueous solutions, e.g., amino acids dissolved in acetone.
![]()
Question 7.
State the properties of a solution.
Answer:
Properties of a solution are as follows:
(a) A solution is a homogeneous mixture.
(b) Particles of a solution are smaller than 1 nm. They cannot be seen with naked eyes.
(c) Particles do not scatter a beam of light.
(d) Solute particles cannot be separated from the mixture by the process of filtration and thus, solution is stable.
Question 8.
Why is crystallisation better than evaporation?
Answer:
Crystallisation is better than evaporation because during evaporation:
(a) some solids, like sugar, are decomposed on heating to dryness.
(b) some impurities may remain dissolved in the solution even after filtration, which on evaporation, contaminates the solid.
Question 9.
What is a suspension? Give examples.
Answer:
Suspension is a heterogeneous mixture of two or more substances. In suspension, particles are suspended throughout the bulk and can be seen with naked eyes. In suspension, particles of solute do not dissolve and are rather suspended. Particles of suspension are large enough to scatter the rays of light and hence, the path of light is visible through a suspension.
Example of suspension – mixture of chalk powder and water, muddy water, mixture of flour and water, mixture of dust particles and air, etc.
Question 10.
What are colloidal solutions? Give examples.
Answer:
Colloidal solution is a heterogeneous mixture of two or more substances. Colloidal solutions appear homogeneous because of relatively small size of particles in comparison with suspension. Particles of colloidal solution are called colloid. Colloids are dispersed throughout the solvent. Particles of colloidal solution are not visible to naked eye but scatter the ray of light, i.e., show Tyndall effect. The size of the molecules of the solute, also called dispersed phase, is between 1 nm and 1000 nm and the molecules remain suspended in the dispersing medium. Colloids cannot be separated by filtration but can be separated using centrifugation. Milk, ink, blood, solution of soap or detergent, etc., are some common examples of colloidal solution.
Question 11.
State the characteristics of colloids.
Answer:
Colloids exhibit the following characteristics:
(a) Brownian motion: Colloidal particles move randomly in zigzag paths like gas particles. This is called Brownian motion. This type of motion is caused due to the collisions between the particles of the dispersion medium and the dispersed phase.
(b) Tyndall effect: When a strong beam of light is passed through a colloidal solution taken in a beaker placed in a dark room, the path of light through the colloidal solution becomes visible. This is called Tyndall effect. The particles become illuminated because they scatter the light falling on them in all directions.
(c) Charge: Colloidal particles carry charge. When electric current is passed through a colloidal solution, the particles move either towards the positive (+) or the negative (-) electrode. This phenomenon is known as electrophoresis. Using the direction of the movement of colloidal particles, we can know the nature of the charge on them.
Question 12.
State the principle of the process of centrifugation.
Answer:
Sometimes, the solid particles in a liquid are very small and pass through a filter paper. For such particles the filtration techniques cannot be used for separation. Such mixtures are separated by centrifugation. The denser particles are forced to the bottom and the lighter particles stay at the top when spun rapidly. The mixture is taken in a closed bottle and rotated at a high speed. The heavy particles settle at the bottom while lighter particles remain behind. For example, to separate cream from milk, milk is churned for 2 – 3 minutes. Cream being lighter than milk, floats at the top of the mixture.
Question 13.
What is chromatography? State its applications.
Answer:
Chromatography is the technique whereby different soluble components of a mixture are separated due to their differential movement over a stationary phase under the influence of a mobile phase, i.e., solvent. If water is the solvent, then the component that is more soluble in water will move faster. In this way, the different components get separated. The technique of chromatography is used to separate:
(a) components of dyes
(b) pigments from natural colours
(c) amino acids
(d) sugar from urine samples
(e) drugs in blood
Question 14.
What is decantation? Explain.
Answer:
Decantation is the process of separating insoluble solids from liquids. A suspension of solid particles in a liquid is allowed to stand for a few minutes. Insoluble solid particles settle down at the bottom due to their weight. This forms the sediment. The clear liquid is then transferred into another container, without disturbing the settled particles. In other words, clear liquid is decanted and separated from the solid.
![]()
Question 15.
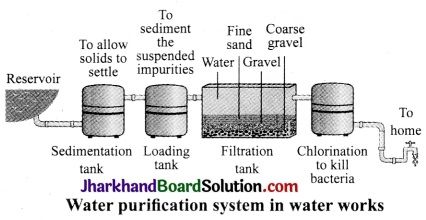
Draw a labelled diagram of water purification system in water works.
Answer:
A flow – diagram of a typical water purification system is given below:
Analysing & Evaluating Questions
Question 16.
The table given below shows the number of grams of five different solids dissolving in 100 g of the solvents: water, alcohol and
chloroform (all at 20°C).
| Solvent | Salt | Sugar | Iodine | Chalk | Urea |
| Water | 36.0 | 204.0 | 0.6 | 0.0 | 100.0 |
| Alcohol | 0.0 | 0.0 | 20.0 | 0.0 | 16.0 |
| Chloro | 0.0 | 0.0 | 3.0 | 0.0 | 0.0 |
(a) Which solid dissolves best in water at 20°C?
(b) Which solid has maximum solubility in alcohol?
(c) Which solid is insoluble in all three solvents?
Answer:
(a) Sugar dissolves best in water at 20°C.
(b) Iodine dissolves maximum in alcohol.
(c) Chalk is insoluble in all the three solvents.
Question 17.
The teacher instructed three students ‘A’, ‘B’ and ‘C’ respectively, to prepare a 50% (mass by volume) solution of sodium hydroxide (NaOH). ‘A’ dissolved 50g of NaOH in 100 mL of water, ‘B’ dissolved 50 g of NaOH in 100 g of water while ‘C’ dissolved 50 g of NaOH in water to make 100 mL of solution. Which one of them has made the desired solution and why?
Answer:
‘C’ has made the desired solution. Mass by volume percentage
\(⇒\frac{\text { Mass of solute }}{\text { Volume of solution }} \times 100\)
\(⇒\frac{50}{100} \times 100=50 \%\)
Long Answer Type Questions
Question 1.
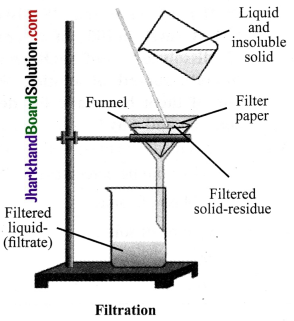
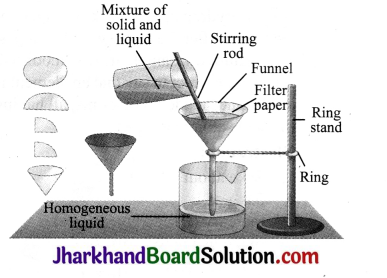
Describe the process of filtration.
Answer:
Filtration is a process by which insoluble solids can be removed from a liquid using a filter paper. A filter paper is a special type of paper which has pores that are tiny enough to let only liquids pass through it. If you pass a solution through filter paper, the undissolved solid particles will be left behind on the paper whereas the liquid will pass through the filter paper. The liquid that passes through is called the filtrate and the undissolved solid particles left behind are called residue.For example, a mixture of chalk powder and water can be separated by this method.
Experiment Set up the apparatus as shown in the figure. The solution is passed through filter paper fitted in the funnel. During filtration, insoluble solid substance is retained in the filter paper as residue while the liquid, free from any suspended matter, passes through the filter paper and is collected as filtrate. This filtrate may be heated to dryness to obtain soluble component.
Question 2.
Describe the method of nitration with the help of a diagram to separate a mixture of two immiscible liquids- kerosene and water.
Answer:
When two liquids do not mix, they form two separate layers and are known as immiscible liquids. These two liquids can be separated using a separating funnel. Experiment: A separating funnel is a special type of glass funnel, which has a stop – cock in its stem to regulate the flow of liquid. It will separate the immiscible liquids into two distinct layers depending on their densities. The heavier liquid forms the lower layer while the lighter one forms the upper layer. Open the stopcock of separting funnel and let the liquid forming lower layer run into the a beaker. Close the stopcock as the liquid forming upper layer reaches the stopcock. You will be left behind with just the liquid forming upper layer in the funnel. Collect this liquid into another beaker. For example, a mixture of kerosene and water is separated by using separating funnel method. This method is also used to extract iron from its ore.
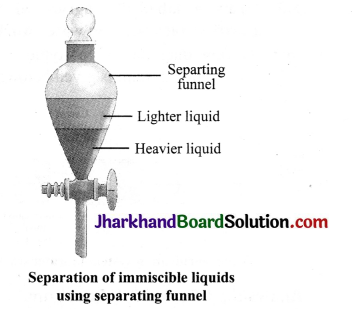
Separation of immiscible liquids using separating funnel
Question 3.
Explain sublimation process with a labelled diagram.
Answer:
Sublimation is the property of a substance in which it is converted directly from solid to gaseous state. Such substances are known as sublime. Some examples of solids which sublime are ammonium chloride, camphor, naphthalene and anthracene. Following is an activity to separate a mixture of ammonium chloride and salt.
Experiment: Take a mixture of ammonium chloride and salt in a china dish and cover it with an inverted conical transparent funnel. At the other end of the funnel, put a cotton plug so that vapours could not come out. Now place the china dish on a burner. As the ammonium chloride is sublime, after heating, it will be converted directly into vapours and this vapours will again condense at the upper colder part of the funnel to form solid ammonium chloride. In this way, the mixture of ammonium chloride and salt can be separated by the
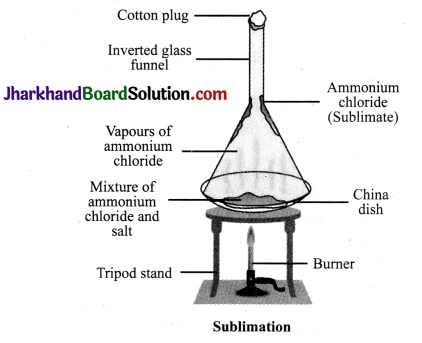
funnel to form solid ammonium chloride. In this way, the mixture of ammonium chloride and salt can be separated by the sublimation method.
Question 4.
Define the term ‘element’. Give an illustration in support of your definition.
Answer:
An element is a substance which contains atoms of the same kind. It cannot be converted into anything visibly simpler than itself. For example, the element mercury is composed of only one kind of atoms with atomic number 80. Gold, copper, hydrogen are other examples of elements. After the discovery of isotopes, the definition of an element is modified. It is more correct to say that an element is a substance made up of atoms, all having the same atomic number.
Illustration:
1. Take about 5.0 g of mercury (II) oxide in a hard glass test tube fitted with cork and delivery tube.
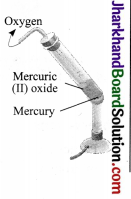
2. Heat the tube over a Bunsen humer flamc first slowly and then strongly. Afier sometime you would observe a gas coming out of che capillary tube.
3. If you bring a glowing maichstick near the mouth of the tube, the slick starts burning with a flame. That is due to esolsed oxygen which is a supporter of combustion,
4. Continue heating the glass lube. After sometime, no more gas is evolved and a shining liquid is left at the bottom of the glasscube. That is mcrcury.
Mercury (II) oxide → Mercury + Oxygen
Conclusion: Rcd mercuric oxide on heating breaks up into two simpler substances, mercury and oxygen which are not further broken. Thus, mercury and oxygen arc elements.
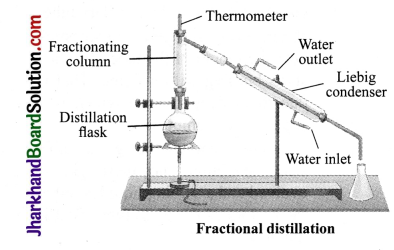
Question 5.
What is a fractionating column? Esplain Its advantage.
Answer:
In case, the difference in the boiling points of the hquids is less than 25 K, WC USC the fractional distillation method. The apparatus is almost the same as used in distillation. hie only diffcrencc is that a fractionating column is tiLted in the distillation flask and the condenser is attached to che fractionating column. A simple fractionating column is made up of a tube packcd with glass beads. The beads provide the surface for the vapours to cool and condense again and again. The fractionating columns obstruct the smooth upward flow of vapours. For example, a mixture of n-hexane and n – heptane can be separated through the process of fractional distillation.
Experiment:
Put the mixture into a distillation flask. Heat the mixture. The vapours of n-hexane has a lower boiling point, so they pass through and get condensed, n – heptane, which has a higher boiling point, condenses and flows back into the distillation flask.
Analysing & Evaluating Questions
Question 6.
Iron filings and sulphur were mixed together and divided into two parts, ‘A’ and ‘B’ Part ‘A’ was heated strongly while Part ‘B’ was not heated. Dilute hydrochloric acid was added to both the parts and evolution of gas was seen in both the cases. How will you identify the gases evolved?
Answer:
Part ‘A’ of the mixture on heating gives iron (II) sulphide.
Fe+S FeS Part ‘B’ of the mixture contains only sulphur and iron filings.
On reactions with dii. HCL the gases given by the two samples are:
Part ‘A’: H2S
(i) Smell of rotten eggs
FeS + 2HCl(aq) → FeCI2(aq) + H2S(g)
(ii) Turns lead acetate paper black
H2S+Pb(CH3COO)2 → PbS + 2CH3COOH
Part ‘B’: H2
(i) Burns with a blue flame and popping sound.
Activity 1
- Divide the class into four groups A, B, C and D.
- Distribute the following samples to each group:
- Few crystals of copper sulphate to group A.
- One spatula full of copper sulphate to group B.
- Chalk powder to group C.
- Few drops of milk or ink to group D.
- Each group should add the given sample in water and stir properly using a glass rod. Are the particles in the mixture visible?
- Direct a beam of light from a torch through the beaker containing the mixture and observe from the front. Is the path of the beam of light visible?
- Leave the mixture undisturbed for a few minutes and set up the filtration apparatus in the meantime.
- Is the mixture stable or do the particles begin to settle after sometime?
- Filter the mixture. Is there any residue on the filter paper?
- Discuss the results and form an opinion.
- Particles of mixture are visible only in case of group C.
- The path of beam of light was visible in case of groups C and D.
- The particles settle down after sometime in case of group C.
- Residue will be left in case of group C. Groups A and B have got a solution. Group C has got a suspension. Group D has got a colloidal solution.
Activity 2
- Fill a beaker half with water.
- Put a watch glass on the mouth of the beaker containing water as shown in the figure.
- Put a few drops of ink on the watch glass.
- Now start heating the beaker. We do not want to heat the ink directly. You will see that evaporation is taking place from the watch glass.
- Continue heating as the evaporation goes on and stop heating when you do not see any further change on the watch glass.
- Observe carefully and record your observations.
- What do you think has got evaporated from the watch glass?
- Is there a residue on the watch glass?
- What is your interpretation? Is ink a single substance (pure) or is it a mixture?
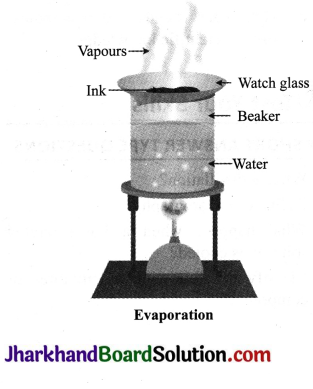
Observations
- Water and volatile substances get evaporated from the watch glass.
- Residue is obtained on the watch glass.
- Ink is a mixture of a dye in water.
Activity 3
- Take a thin strip of filter paper.
- Draw a line on it using a pencil, approximately 3 cm above the lower edge.
- Put a small drop of ink (water – soluble, that is from a sketch pen or fountain pen) at the centre of the line. Let it dry.
- Lower the filter paper into jar/glass/beaker/ test tube containing water so that the drop of ink on the paper is just above the water level and leave it undisturbed.
- Watch carefully, as the water rises up on the filter paper. Record your observations.
- What do you observe on the filter paper as the water rises on it?
- Do you obtain different colours on the filter paper strip?
- What according to you, can be the reason for the rise of the coloured spot on the paper strip?
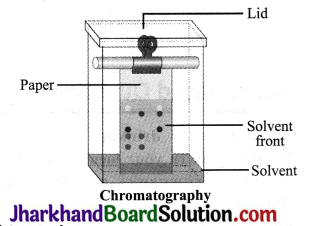
Observations
- With water, ink also rises up, though with smaller speed.
- Different colours will be observed on the filter paper.
- Ink is a mixture of different coloured substances. Each substance moves with a different characteristic speed with respect to the speed of water.
Activity 4
- Take some (approximately 5 g) impure sample of copper sulphate in a china dish.
- Dissolve it in minimum amount of water.
- Filter the impurities out.
- Evaporate water from the copper sulphate so as to get a saturated solution.
- Cover the solution with a filter paper and leave it undisturbed at room temperature to cool down slowly, for a day.
- You will obtain the crystals of copper sulphate in the china dish.
- This process is called crystallisation.
- What do you observe in the china dish?
- Do the crystals look alike? How will you separate the crystals from the liquid in the china dish?
Observations
- The crystals of copper sulphate are obtained in the china dish.
- The crystals look alike to some extent.
- Crystals can be separated from the liquid by filtration. When the liquid passes through the funnel, crystals are retained on the filter paper.
Value Based Questions
Question 1.
Purification of drinking water is a big problem. In your locality, vendors of various companies are promoting RO units for purificaiton of water for domestic use irrespective of whether it is required or not. Answer the following questions:
(a) What is the meaning of Ro?
(b) Would you recommend the installation of Ro unit in all the areas? Give reason.
(c) What values are associated with your approach?
Answer:
(a) RO means reverse osmosis.
(b) Installation of RO units for purifying the drinking water in all the areas is not recommended. An RO plant generally removes about 95% of TDS (total dissolved salts) in water that is treated by RO. The desirable level of TDS as per Indian standard is 500 ppm. Before installing RO unit, it is important to check the TDS level of your input water.
(c) Awareness of drinking TDS in potable water supply to the people.
![]()
Question 2.
After cleaning the refrigerator thoroughly well, Rohit closed it and kept it switched off for two days. After that, on opening it, he got a foul smell. His neighbourer advised him to keep a piece of charcoal (C) in the refrigerator.
(a) As a student of chemistry, explain why this suggestion is effective?
(b) Name another substance which can be used in place of charcoal.
(c) What values can be drawn from this advice?
Answer:
(a) The charcoal piece absorbs the foul – smelling gases developed in refrigerator.
(b) Coal.
(c) Help your friends and neighbours/ use of scientific knowledge.