JAC Board Class 9th Science Chapter 1 Notes Matter in Our Surroundings
→Matter: Everything in this universe is made up of material which is called matter. Matter is anything that has mass and occupies space. Matter is made up of lots of tiny particles.
→ Characteristics of particles of matter:
a. Particles of matter have space between them.
b. Particles of matter are continuously moving.
c. Particles of matter attract each other. Matter exists in three different states, viz., solid, liquic and gas.
| Solid | Liquids | Gas |
| 1. Strong intermolecular force of attraction. | 1. Weak intermolecular force of attraction. | 1. Very weak intermolecular force of attraction. |
| 2. Very less intermolecular space. | 2. Large intermolecular space. | 2. Very large intermolecular space. |
| 3. Have definite shape and volume. | 3. No definite shape but definite volume. | 3. No definite shape and volume. |
| 4. High density, high melting and boiling points. | 4. Density is lower, low melting and boiling points. | 4. Density is very low. |
| 5. Solids cannot be compressed. | 5. Liquids can be compressed. | 5. Gases are highly compressible. |
| 6. Solids cannot flow. | 6. Liquids can flow. | 6. Gases can flow. |
→ Matter can change its state from solid to liquid and from liquid to gas and vice-versa.
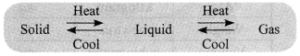
→ Effect of Temperature: On increasing temperature, the particles gain energy and start vibrating with greater energy. Due to increased kinetic energy, the particles overcome the force of attraction and a new state is obtained.
→ Melting Point: It is the temperature at which a solid becomes a liquid at atmospheric pressure. Boiling Point: It is the temperature at which a liquid changes into its vapour form at atmospheric pressure.
![]()
→ Latent Heat of Fusion: The amount of heat energy required to change 1 kg of a solid into liquid at its melting point is called the latent heat of fusion of the solid.
→ Latent Heat of Vaporisation: The amount of heat energy required to change 1 kg of a liquid into vapour at atmospheric pressure, at its boiling point is called the latent heat of vaporisation of the liquid.
→ Sublimation: It is the change of state directly from solid to gas or vice-versa without going through the liquid state. Evaporation: It is a surface phenomenon in which a liquid changes into vapour/gas below its boiling point. It results in lowering of temperature, i.e., cooling is caused when evaporation takes place.
→ Factors affecting Evaporation: An increase in surface area increases the rate of evaporation. An increase in temperature increases the rate of evaporation. A decrease in humidity increases the rate of evaporation. An increase in wind speed increases the rate of evaporation.
→ Some measurable quantities and their units:
| Quantity | SI Unit | A Symbol |
| Temperature | kelvin | K |
| Length | metre | m |
| Mass | kilogram | kg |
| Weight | newton | N |
| Volume | cubic metre | m3 |
| Density | kilogram per cubic metre | kg/m3 |
| Pressure v | pascal | Pa |