JAC Board Class 9th Science Solutions Chapter 3 Atoms and Molecules
JAC Class 9th Science Atoms and Molecules InText Questions and Answers
Page 32
Question 1.
In a reaction, 5.3g of sodium carbonate reacted with 6g of ethanoic acid. The products were 2.2g of carbon dioxide, 0.9g water and 8.2g of sodium ethanoate. Show that these observations are in agreement with the law of conservation of mass. Sodium carbonate + Acetic acid → Sodium acetate + Carbon dioxide + Water
Answer:
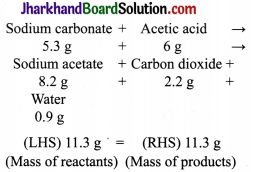
This shows that during a chemical reaction, mass of reactants mass of products. Hence the given observation are in agreement with the law of conservation of mass.
Question 2.
Hydrogen and oxygen combine in the ratio of 1 : 8 by mass to form water. What mass of oxygen gas would be required to react completely with 3g of hydrogen gas?
Answer:
Ratio of H : O by mass in water is:
Hydrogen : Oxygen → H2O ⇒ 1 : 8 = 3 : x or x = 24g
24 g of oxygen gas would be rcquircd to react completely with 3g of hydrogen.
Question 3.
Which postulate of Dalton’s atomic theory is the result of the Law of consersatlon of mass?
Answer:
Awms are indivisible particles, which can neither be created nor destroyed in a chemical rcacuon.
Question 4.
Which postulate of Dalton’s atomic theory can explain the law of definite proportions?
Answer:
“The relative number and kind of atoms are constant in a given compound”
Page 35
Question 1.
Define the atonik mass unit.
Answer:
A unit of mass used in express atomic and molecular weights, equal to one twelfth (1/12th.) of the mass of an stom of carbon – 12 The relative atomic masses of all elements have been found with respect to an atom of carbon – 12.
Question 2.
Why Is it not possible to see an atom with naked eyes?
Answer:
The size of an atom is very small, Further, atoms of most elements do not exist independently. The radius of an atom is of the order of 10-10m.
Page 39
Question 1.
Write down the formulae of
(a) sodium oxide
(b) aluminium chloride
(c) sodium sulphide
(d) magnesium hydroxide
Answer:
(a) Sodium oxide — Na2O
(b) Aluminium chloride — AlCl3
(c) Sodium sulphide — Na2S
(d) Magnesium hydroxide — Mg(OH)2
Question 2.
Write down the names of compounds represented by the following formulae:
(a) Al2(SO4)3
(b) CaCl2
(c) K2SO4
(d) KNO3
(e) CaCO3
Answer:
(a) Al2(SO4)3 : Aluminium sulphate
(b) CaCl2 : Calcium chloride
(c) K2SO2 : Potassium sulphate
(d) KNO3 : Potassium nitrate
(e) CaCO2 : Calcium carbonate
Question 3.
What Is meant by the term chemical formula?
Answer:
The chemical formula of a compound is the symbolic representation of its composition. It gives the number and kinds of atoms which are chemically united in a given compound. For example, chemical formula of sodium chloride is NaCl.
Question 4.
How many atoms are present in a
(a) H2S molecule and
(b) \(\mathrm{PO}_{4}^{3-}\) ion?
Answer:
(a) H2S → 3 atoms are present: 2 atoms of hydrogen and 1atom of sulphur.
(b) \(\mathrm{PO}_{4}^{3-}\) → 5 atoms are present: 1 atom of phosphorus and 4 atoms of oxygen.
Page 40
Question 1.
Calculate the molecular masses of H2, O2 C12, CO2, CH4, C2H6, C2H4, NH3, CH3OH.
Answer:
The molecular masses are:
H2 → 1 + 1 = 2u
O2 → 16 + 16 = 32u
Cl2 → 35.5 + 35.5 = 71u
CO2 → p 12 + 32 = 44u
CH4 → 12 + 4 = 16u
C2H6 → (12 × 2) + (1 × 6) = 30u
C2H4 → (12 × 2) + (1 × 4) = 28u
NH3 → (1 × 14) + (1 × 3) = 17u
CH3OH → (1 × 12) + (1 × 3) + (1 × 16)(1 × 1) = 32u.
Question 2.
Calculate the formula unit of masses of ZnO, Na2O, K2CO3, given atomic masses of Zn = 65u, Na = 23u, K = 39u, C = 12u and O = 16u.
Answer:
The formula Unit mass of:
(a) ZnO = 65u + 16u = 81u
(b) Na2O = (23u × 2) + 16u = 46u + 16u = 62u
(c) K2CO3 = (39u × 2) + 12u + (16u × 3)
= 78u + 12u + 48u
= 138u.
Page 42
Question 1.
If 1 mole of carbon atoms weigh 12grams, what Is the mass (in grams) of 1 atom of carbon?
Answer:
1 mole of carbon atoms = 6.022 × 1023 atoms
Now, 12/6.022 × 1023 atoms of carbon weigh = 12g
One atom of carbon weighs = \(\frac{12}{6.023}\) × 1023
= 1.99 × 10-23g.
Question 2.
Which has more number of atoms 100 grams of sodium or loo grams of iron (given atomic mass of Na = 23u, Fe 56u)?
Answer:
23 gram atomic unit or 23g sodium (1 mole) = 6.022 × 1023 atoms
100 gram atomic unit or 100g sodium = \(\frac{6.022 \times 10^{23} \times 100}{23}\)
= 2.617 × 1024 atoms Again 56 gram atomic unit or 56 g iron (1 mole) 6.022 × 1023
100 gram atomic unit or 100 g iron = \(\frac{6.022 \times 10^{23} \times 100}{56}\)
= 1. 075 × 1024 atoms Thus, 100 g of sodium has more atoms than 100g of iron
JAC Class 9th Science Atoms and Molecules Textbook Questions and Answers
Question 1.
A 0.24g sample of compound of oxygen and boron was found by analysis to contain 0.096g of boron and 0.144g of oxygen. Calculate the percentage composition of the compound by weight.
Answer:
Percentage (%) of boron in the sample
\(\frac{0.096}{0.24}\) × 100 = 40%
Percentage (%) of oxygen in the sample
\(\frac{0.144}{0.24}\) × 100 = 60%
The sample of compound contains 40% boron and 60% oxygen by weight.
Question 2.
When 3.0g of carbon is burnt in 8.00 g oxygen, 11.00g of carbon dioxide is produced. What mass of carbon dioxide will be formed when 3.00g of carbon Is burnt In 50.00g of oxygen? Which law of chemical combination will govern your answer?
Answer:
When 3.0g of carbon is burnt in 8.00g oxygen, 11.00g carbon dioxide is produced. It means all of carbon and oxygen are used up and carbon and oxygen are combined in the ratio of 3 : 8 to form carbon dioxide. Thus when there is 3g carbon and 50.0g oxygen, then also only 8g oxygen will be used and 11.0g carbon dioxide will be formed. The remaining oxygen is not used up. This indicates law of defmite proportions which Say that in compounds, the combining elements are present in definite proportions by mass.
Question 3.
What are polyatomic ions? Give examples.
Answer:
The ions which contain more than one atom (same kind or may be of different kind) and behave as a single unit are called polyatomic ions. For example:
- Ammonium ion NH is a compound ion which is made up of two types of atoms joined together, viz., nitrogen and hydrogen.
- Carbonate ion CO is a compound ion which is made up of two types of atoms joined together, viz., carbon and oxygen.
Question 4.
Write the chemical formulae of the following:
(a) Magnesium chloride
(b) Calcium oside
(c) Copper nitrate
(d) Aluminium chloride
(e) Calcium carbonate
Answer:
(a) Magnesium chloride : MgCl
(b) Calcium oxide : CaO
(e) Copper nitrate : Cu(NO3)2
(d) Aluminium chloride : AlCl3
(e) Calcium carbonate: CaCO3
Question 5.
Give the names of the elements present in the following compounds:
(a) Quick lime
(b) Hydrogen bromide
(c) Baking powder
(d) Potassium sulphate
Answer:
(a) Quick lime : Calcium and oxygen
(b) Hydrogen bromide : Hydrogen and bromine
(c) Baking powder : Sodium, hydrogen. carbon and oxygen
(d) Potassium sulphate : potassium. sulphur and oxygen
Question 6.
Calculate the molar mass of the following substances.
(a) Ethvne, C2H2
(b) Sulphur molecule, S8
(c) Phosphorus molecule, P4 (Atomic mass of phosphorus = 31u)
(d) Hydrochloric acid, HCl
(e) Nitric acid, HNO3
Answer:
(a) Ethyne, C2H2 = (2 × 12) + (2 × 1) = 26g
(b) Sulphur molecule, S8 = 8 × 32 = 256g
(c) Phosphorus molecules, P4 = 4 × 31 = 124g
(d) Hydrochloric acid, HCl = (1 × 1) + (1 × 35.5) = 36.5g
(e) Nitric acid, HNO3 = (1 × 1) + (1 × 14) + (3 × 16) = 63g
Question 7.
What is the muss of
(a) 1 mole of nitrogen atoms?
(b) 4 moles of aluminium atoms (atomic mass of aluminium 27u)?
(c) 10 moles of sodium sulphite (Na2SO3)?
Answer:
(a) 1 mole of nitrogen atoms 14u = 14g
(b) 4 moles of aluminium atoms = 4 × 27 = 108u = 108g
(c) 1 mole of sodium sulphite = (2 × 23) + (1 × 32) + (3 × 16) = 126u = 126g
10 moles of sodium sulphite = 10 × 126 = 1260g.
Question 8.
Convert Into mole.
(a) 12g of oxygen gas
(b) 20g of water
(c) 22 g of carbon dioxide
Answer:
(a) Given mass of oxygen gas = 12g
Molar mass of oxygen gas (O2) = 32g
Mole of oxygen gas = \(\frac{12}{32}\)
= 0.375 mole.
(b) Given mass of water = 20g
Molar mass of water(H2O) (2 × 1) + 16= 18g
Mole of water = \(\frac{20}{18}\)= 1.11 mole.
(c) Given mass of carbon dioxide = 22g
Molar mass of carbon dioxide (CO2) (1 × 12) + (2 × 16) = 12 + 32 = 44g
Mole of carbon dioxide = \(\frac{22}{44}\)
= 0.5 mole.
Question 9.
What is the mass of
(a) 0.2 mole of oxygen atoms?
(b) 0.5 mole of water molecules?
Answer:
(a) Mole of oxygen atoms = 0.2 mole
Molar mass of oxygen atoms = 16g
Mass of oxygen atoms 16 × 0.2 = 3.2g.
(b) Mole of water molecule 0.5 mole
Molar mass of water molecules = (2 × 1) + 16 = 18g
Mass of H2O = 18 × 0.5 = 9g.
Question 10.
Calculate the number of molecules of sulphur (S8) present In 16 g of solid sulphur.
Answer:
Molar mass of sulphur (S8) = 256 g = 6.022 × 1023 molecules
Given mass of sulphur = 16g
Molecules of sulphur = \(\frac{16 \times 6.022 \times 10^{23}}{256}\)
\(=\frac{96.35 \times 10^{23}}{256}\) = 0.376 × 1023 molecules.
Question 11.
Calculate the number of aluminium ions present In 0.051 g of aluminium oxide.
Hint: The mass of an ion is the same as that of an atom of the saine element Atomic mass of Al = 27u
Answer:
1 mole of aluminium oxide, Al2O3 = (2 × 27) + (3 × 16) = 102u = 102g
102 g of Al2O3 has = 6.022 × 1023 Al2O3 molecules
0.051 g of Al2O3has = \( \frac{6.022 \times 10^{23} \times 0.051}{102}\)
= 3.01 × 1020 molecules
1 molecule of Al2O3 gives = 2 Al3+ ions
Hence, 0.051g Al2O3 gives = 2 × 3.01 × 1020 Al3+ ions
= 6.022 × 1020 aluminium ions.