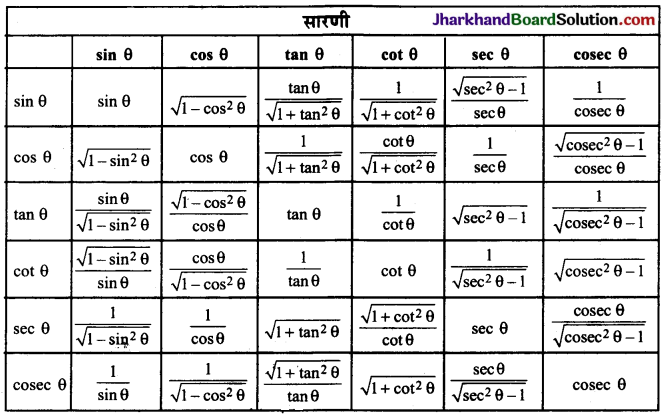
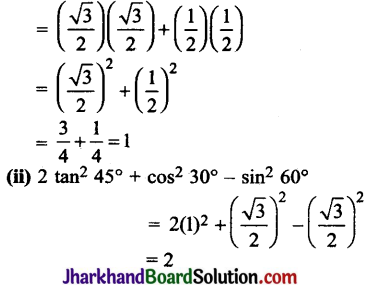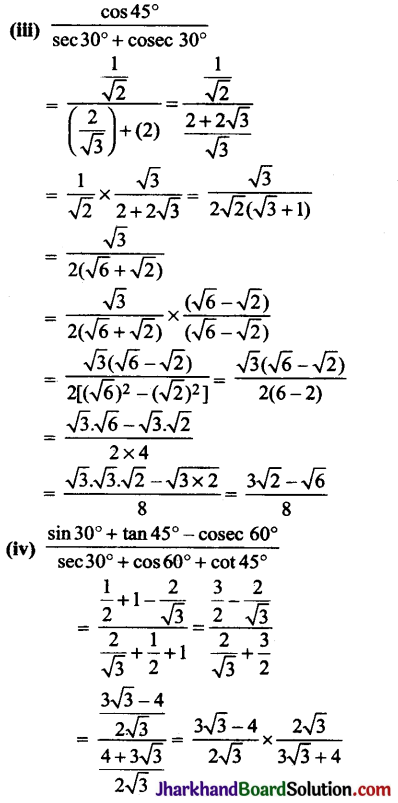Jharkhand Board JAC Class 10 Science Solutions Chapter 4 Carbon and Its Compounds Textbook Exercise Questions and Answers.
JAC Board Class 10 Science Solutions Chapter 4 Carbon and Its Compounds
Jharkhand Board Class 10 Science Carbon and Its Compounds Textbook Questions and Answers
Question 1.
Ethane, with the molecular formula C2H6 has …
(a) 6 covalent bonds.
(b) 7 covalent bonds.
(c) 8 covalent bonds.
(d) 9 covalent bonds.
Answer:
7 covalent bonds.
Question 2.
Butanone is a four carbon compound with the functional group ….
(a) carboxylic acid.
(b) aldehyde.
(c) ketone.
(d) alcohol.
Answer:
ketone.
Question 3.
While cooking, if the bottom of the vessel is getting blackened on the outside, it means that…
(a) the food is not cooked completely.
(b) the fuel is not burning completely.
(c) the fuel is wet.
(d) the fuel is burning completely.
Answer:
the fuel is not burning completely.

Question 4.
Explain the nature of the covalent bond using the bond formation in CH3Cl.
Answer:
The atomic numbers of carbon, hydrogen and chlorine are 6, 1 and 17 respectively. Hence, their electronic configuration may be written as follows :
| Atom | K shell | L shell | M shell |
| Carbon | 2 | 4 | – |
| Hydrogen | 1 | – | – |
| Chlorine | 2 | 8 | 7 |
- This indicates that, carbon requires 4 electrons to complete its octet; hydrogen requires 1 electron to complete its duplet and chlorine requires 1 electron to complete its octet.
- Therefore, carbon shares its four electrons, one electron each with three hydrogen atoms and one electron with chlorine atom to form four covalent bonds completing its octet.
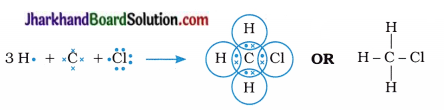
- Thus, carbon attains the stable electronic configuration of nearest noble gas neon, hydrogen attains the electronic configuration of noble gas helium, while chlorine acquires the electronic configuration of noble gas argon.
- In short, chloromethane forms three C – H and one C – Cl covalent bonds.
Question 5.
Draw the electron dot structures for –
(a) Ethanoic acid
(b) H2S
(c) Propanone
(d) F2
Answer:
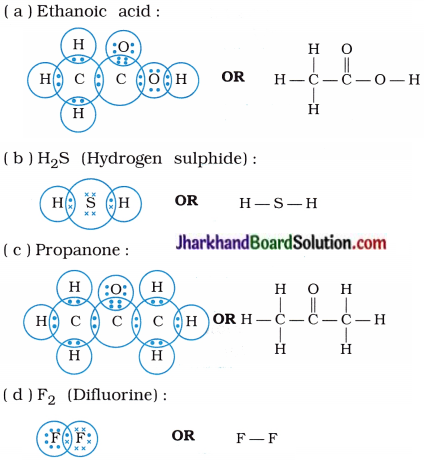
Question 6.
What is an homologous series ? Explain with an example.
Answer:
Definition : A series of compounds in which hydrogen atom / atoms remain in carbon chain are replaced by same functional group, then it constitute the homologous series.
OR
A series of organic compounds in succession which differ by a definite group (like – CH2 -) is called homologous series.
Explanation:
CH3 – OH Methanol
CH3 – CH2 – OH Ethanol
CH3 – CH2 – CH2 – OH Propanol
CH3 – CH2 – CH2 – CH2 – OH Butanol
- It is a homologous series of alcohol where each member of the series possesses functional group – OH (Hydroxyl group).
- The difference in molecular formula between two successive members of the series is equal to -CH2-, hence, difference in molecular masses of any two successive members of the series is 14 u.

Question 7.
How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties?
Answer:
Difference in physical properties :
| Physical property | Ethanol | Ethanoic acid |
| 1. Smell | Pleasant (Sweet) | Vinegar-like strong |
| 2. Taste | Burning taste | Sour |
| 3. Melting point (K) | 156 | 290 |
| 4. Boiling point (K) | 351 | 391 |
Difference in chemical properties :
| Reaction with | Ethanol | Ethanoic acid |
| 1. Sodium bicarbonate | CO2 gas does not evolve. | CO2 gas is evolved. |
| 2. Alkaline KMnO4 | Pink colour of KMnO4 disappear. | Pink colour of KMnO4 does not disappear. |
Question 8.
Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?
Answer:
A soap molecule has two ends which possess different properties. One end of soap has a polar head which is called hydrophilic head, while the other end has a non-polar tail which is also called hydrophobic tail.
- Polar head has affinity towards water molecules, while non-polar tail being hydrophobic has no affinity towards water molecules.
- When soap is dissolved in water, the polar head dissolves in water but non-polar tail remains outside the water surface. As a result, spherical micelles are formed.
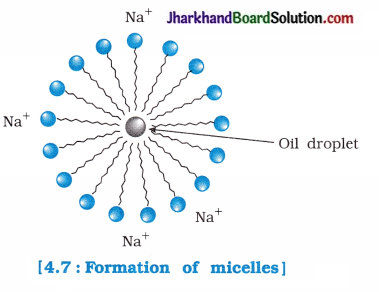
- Since, soap is soluble in ethanol, therefore micelle formation does not take place in ethanol.
Question 9.
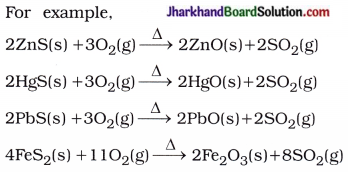
Why are carbon and its compounds used as fuels for most applications?
Answer:
When carbon is burnt in the presence of air or oxygen, it forms carbon dioxide and water with a release of a large amount of heat and light.
C(s) + O2(g) → CO2(g) + Heat + Light
- When carbon and its compounds are burnt, additional heat energy is not required for consistent burning.
- Hence, carbon and its compounds are used as fuels for most applications.
Question 10.
Explain the formation of scum when hard water is treated with soap.
Answer:
Hard water possesses calcium and magnesium ions which combine with soap molecules to form curdy white precipitate of calcium and magnesium salts, which is called scum.
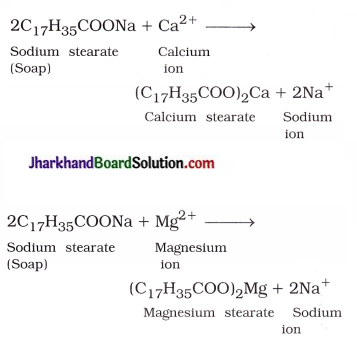
Question 11.
What change will you observe if you test soap with litmus paper (red or blue)?
Answer:
Soap is basic (alkaline) in nature, it turns red litmus paper blue, while, it does not have any effect on blue litmus paper.
Question 12.
What is hydrogenation? What is its industrial application?
Answer:
A reaction in which unsaturated hydrocarbons add hydrogen in the presence of catalysts such as nickel or palladium to form saturated hydrocarbons is known as hydrogenation reaction.
Industrial application : It is used to convert vegetable oil into vegetable ghee.
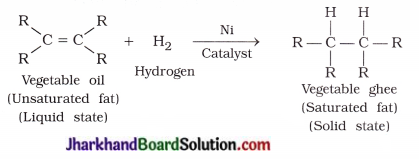
Question 13.
Which of the following hydrocarbons undergo addition reactions?
C3H6, C3H8, C2H2 and CH4
Answer:
C3H6 and C2H2 are unsaturated hydrocarbons, hence, they undergo addition reaction.

Question 14.
Give a test that can be used to differentiate between saturated and unsaturated hydrocarbons.
Answer:
Butter contains saturated fats while cooking oil contains unsaturated fats.
Unsaturated compound decolourise the pink colour of alkaline KMnO4 solution.
When butter is treated with a few drops of alkaline KMnO4 solution, the pink colour of KMnO4 does not disappear, while, when cooking oil is treated with a few drops of alkaline KMnO4 solution, its pink colour disappears.

Question 15.
Explain the mechanism of the cleaning action of soaps.
Answer:
The molecules of soap are sodium or potassium salts of long chain carboxylic acids.
- Two ends of soap molecule possess different properties. One end is hydrophilic and it dissolves in water, while the other end is hydrophobic and it dissolves in hydrocarbons.
- When soap is at the surface of water, the hydrophobic ‘tail’ of soap will not be soluble in water and the soap will align along the surface of water with the ionic end in water and the hydrocarbon ‘tail’ protruding out of water.

- Inside water, these molecules have a particular orientation that keeps the hydrocarbon portion out of the water.
- This happens due to the formation of clusters of molecules in which the hydrophobic tails are in the interior part of the cluster and the ionic ends are on the surface of the cluster.
- This formation is called a micelle.
- Soap in the form of a micelle collects the oily dirt in the centre of it and is able to clean.
- These micelles exist in solution as a colloid.
- Micelles do not cluster to precipitate because of ion-ion repulsion.
- Thus, the dirt suspended in the micelles is also easily rinsed away.
- The soap micelles are large enough to scatter light, hence a soap solution appears cloudy.
Jharkhand Board Class 10 Science Carbon and Its Compounds InText Questions and Answers
Question 1.
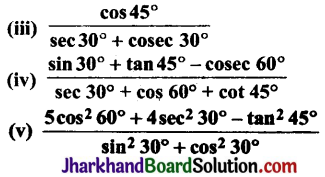
What would be the electron dot structure of carbon dioxide which has the s formula CO2?
Answer:
The electronic configuration of carbon and oxygen are as follows:

Thus, carbon has 4 electrons and oxygen s has 6 electrons in the valence shell.
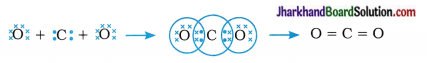
Question 2.
What would be the electron dot structure of a molecule of sulphur which is made-up of eight atoms of sulphur? [Hint: The eight atoms of sulphur are joined together in the form of a ring.]
Answer:
The electronic configuration of 16S is as follows :
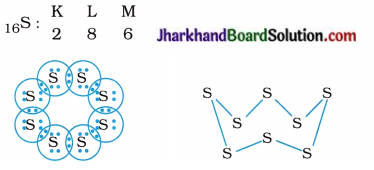
Question 3.
Why is the conversion of ethanol to ethanoic acid an oxidation reaction?
Answer:
The conversion of ethanol to ethanoic acid is shown below:

In this reaction, a molecule of ethanol contains one oxygen atom while ethanoic acid contains two oxygen atoms. Hence, oxygen atom is added during the reaction; therefore, this conversion is an oxidation reaction.
Question 4.
A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
Answer:
Ethyne is an unsaturated hydrocarbon.
- Combustion of ethyne in air produces a yellow flame with lot of black smoke.
- Due to this incomplete combustion, heat produced is very less than the heat required for welding; hence, the mixture of ethyne and air is not used for welding.
- When the combustion of ethyne is carried out with oxygen, due to complete combustion heat produced is very large which is usually needed for welding; hence the mixture of ethyne and oxygen is used for welding.
- 2[CH ≡ CH] + 5O2 → 4CO2 + 2H2O + Heat and Light
Question 5.
How would you distinguish experimentally between an alcohol and a carboxylic acid?
Answer:
An alcohol and a carboxylic acid can be distinguished experimentally by the following tests :
(1) Sodium hydrogencarbonate test: Take a small amount of each substance in two different test tubes and add to it an aqueous solution of sodium hydrogencarbonate.
- Test tube in which brisk effervescence of CO2 gas appear contains carboxylic acid.
- Solution of ethanol does not produce brisk effervescence of CO2 gas.
(2) Alkaline potassium permanganate test:
Take a small amount of each substance in two different test tubes, and add to it a few drops of alkaline potassium permanganate and warm the test tubes.
- The purple colour of potassium permanganate disappear in the test tube containing alcohol.
- Solution of carboxylic acid does not discharge the colour of potassium permanganate.
Question 6.
What are oxidising agents?
Answer:
Substances, which are capable of adding oxygen to other substances are known as oxidising agents. For example, alkaline KMnO4, acidic K2Cr2O7.
Question 7.
Would you be able to check if water is hard by using a detergent?
Answer:
No. Because detergent produces foam in both hard and soft water.

Question 8.
People use a variety of methods to wash clothes. Usually after adding the soap, they ‘beat’ the clothes on a stone, or beat it with a paddle, scrub with a brush or the mixture is agitated in a washing machine. Why is agitation necessary to get clean clothes?
Answer:
Soap decreases the surface tension of water. When the soap is applied to any dirty clothes or on stains, the non-polar tail containing hydrocarbon part is attracted towards the molecule of dirt, oil or grease; while the polar tail part is attracted towards water. Thus, micelles are formed. Hence, to remove the micelles from the surface of the clothes, agaitation of clothes is necesary.
Question 9.
How many structural isomers can you draw for pentane?
Answer:
Structural isomers of pentane (C5H12) :
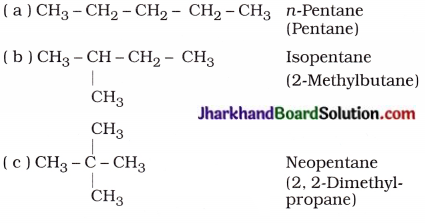
Question 10.
What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
Answer:
Carbon atom forms covalent bonds by sharing of electrons with other atoms to form numerous compounds. The number of carbon compounds known to chemists was recently estimated about three millions. Carbon has ability to form a large number of compounds. Two factors noticed in this case are as follows :
Catenation property of carbon : Carbon has the unique ability to form bonds with other atoms of carbon, giving rise to large number of molecules. This property of carbon is called catenation.
- Carbon compounds consist of long chains of carbon, branched chains of carbon or in the ring form or cyclic form.
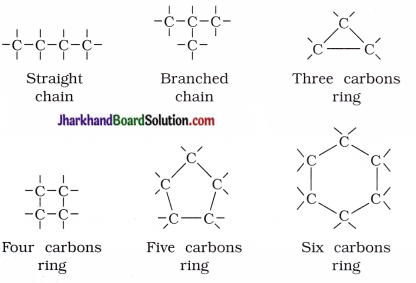
- Carbon atom may be linked with other atoms by single, double or triple bonds.
- Compounds of carbon, which are linked by only single bonds between the carbon atoms are called saturated compoundsr
- Compounds of carbon, having double or triple c bonds between the carbon atoms are called unsaturated compounds.
- No other element exhibits the property of catenation to the extent seen in carbon compounds. Silicon forms compounds with hydrogen which have chains of upto seven or eight atoms, but these compounds are very reactive.
- The carbon-carbon bond is very strong and hence it is stable.
- Since carbon has a valency of four, it is capable of bonding with four other atoms of carbon or atoms of some other monovalent elements.
- Compounds of carbon are formed with oxygen, hydrogen, nitrogen, sulphur, chlorine and many other elements giving rise to compounds with specific properties which depend on the elements other than carbon present in the molecule.
- The bond formed by carbon with most other elements are very strong, which makes compounds exceptionally stable.
- Carbon being small in size, it enables the nucleus to hold on to the shared pairs of electrons strongly. Hence, strong bonds are formed by carbon.
Question 11.
What will be the formula and electron dot structure of cyclopentane?
Answer:
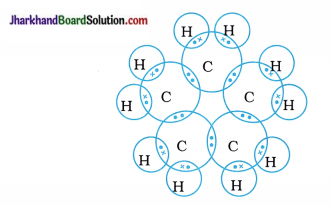
The general formula of cycloalkane is CnH2n; hence, molecular formula of cyclopentane Is C5H10.
The electron dot structure of cyclopentane is shown below:
Question 12.
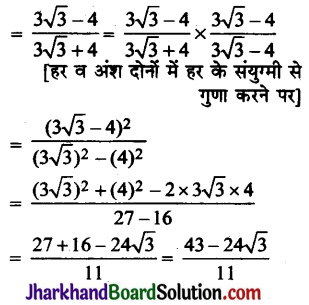
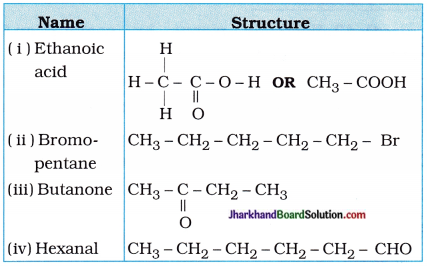
Draw the structures for the following compounds:
(i) Ethanoic acid
(ii) Bromopentane
(iii) Butanone
(iv) Hexanal
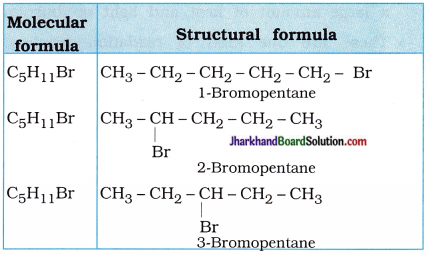
Are structural isomers possible for bromo-pentane?
Answer:
Possible structural isomers of bromopentane are as follows :
Question 13.
How would you name the following compounds?
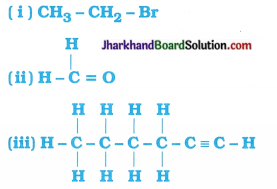
Answer:
The name 0f the given compounds are:
- Bromoethane
- Methanal
- Hex-l-yne or Hexyne.
Activity 4.1 [T.B. Pg. 58]
Aim : To demonstrate that most of the things we use in our day to day life consists of carbon.
Questions:
- Make a list of ten things you have used or consumed since morning.
- Compile this list with the lists made by your s classmates and then sort the items into the? adjascent table.
If there are items which are made-up of? more than one material, put them into both < the relevant columns.
Answer:
A list of ten things used or consumed s since morning is given below:
Toothbrush, toothpaste, bucket, water, soap, S detergent, cooking utensils, cup, milk, medicine, S’ newspaper, books.
| Things made of metal | Things made of glass / clay | Others |
| Cooking utensils, bucket | Cup | Toothbrush, toothpaste, water, soap, detergent, milk, medicine, news-paper, books. |
Activity 4.2 [T. B. Pg. 67]
Aim : To study the concept of homologous series.
Questions:
Question 1.
Calculate the difference in the formulae and molecular masses for (a) CH3OH and C2H5OH, (b) C2H5OH and C3H7OH and (c) C3H7OH and C4H9OH
Answer:
| Difference in formulae | Difference in molecular masses |
| (a) C2H5OH – CH3OH = CH2 | [2(12) + 6(1) +1(16)] – [1(12) + 4(1) + 1(16)] = 46 – 32 = 14 u |
| (b) C3H7OH – C2H5OH = CH2 | [3(12) + 8(1)+1(16)] – [2(12) + 6(1) + 1(16)] = 60 – 46 = 14 u |
| (c) C4H9OH- C3H7OH = CH2 | [4(12) + 10(1) + 1(16)] – [3(12) + 8(1) + 1(16)] = 74 – 60 = 14 u |
Question 2.
Is there any similarity in these three compounds mentioned above?
Answer:
Similarity observed in above three compounds are as follows :
- Difference in molecular formulae of these compounds is CH2.
- Difference in molecular masses of these compounds is 14 u.
- In the nomenclature of these compounds, a common suffix -ol is written.
- These compounds are represented by the general formula CnH2n+1OH.
- Chemical reactions of these compounds are identical.
- All the three compounds are alcohols.
Question 3.
Arrange these alcohols in the order of increasing carbon atoms to get a family. Can we call this family a homologous series?
Answer:
Alcohols in the increasing order of carbon atoms are as follows:
CH3OH, C2H5OH, C3H7OH, C4H9OH
Yes, we call this family a homologous series.

Question 4.
Generate the homologous series for compounds containing up to four carbons for the other functional groups given in Q. 30.
Answer:
Homologous series for halogen group : [CnH2n+1X]
CH3Cl, C2H5Cl, C3H7Cl, C4H9Cl
→ Homologous series for aldehyde group : [CnH2nO]
HCHO, CH3CHO, CH3CH2CHO, CH3CH2CH2CHO
→ Homologous series for ketone group : [CnH2nO]
CH3COCH3, CH3COC2H5, CH3COC3H7, CH3COC4H9
→ Homologous series for carboxylic acid group : [CnH2nO2]
HCOOH, CH3COOH, CH3CH2COOH, CH3CH2CH2COOH
Activity 4.3 [T. B. Pg. 69]
Aim : To study the flame of burning of carbon compounds.
Caution:
This activity needs the teacher’s assistance.
Activity:
- Take some carbon compounds (naphthalene, camphor, alcohol) one by one on a spatula t and burn them in the flame.
- Observe the nature of the flame and note s whether smoke is produced.
- Place a metal plate above the flame. Is there a deposition on the plate in case of any of the compounds?
Observation :
- Camphor and alcohol burn with a blue non-luminous flame and there is no sooty deposit on the metal plate.
- Naphthalene burns with a luminous yellow flame and there is sooty deposit on the metal plate.
Inference :
Camphor and alcohol are saturated hydrocarbons, while, naphthalene is an unsaturated hydrocarbon.
Activity 4.4 [T. B. Pg. 69]
Aim: To study the flame formed by complete and incomplete combustion.
Activity:
Light a bunsen burner and adjust the air hole at the base to get different types of flames / presence of smoke.
Questions:
Question 1.
When do you get a yellow flame?
Answer:
When the holes of the bunsen burner are not opened, enough supply of oxygen-rich air is not available, hence, incomplete combustion of hydrocarbon produces a yellow flame.
Question 2.
When do you get a blue flame?
Answer:
When the holes of the bunsen burner are opened, enough supply of oxygen-rich air is available, hence, complete combustion of hydrocarbon takes place and blue flame is formed.
Activity 4.5 [T. B. Pg. 70]
Aim: To study the oxidation of alcohols with alkaline KMnO4.
Activity:
- Take about 3 mL of ethanol in a test tube and warm it gently in a water bath.
- Add a 5 % solution of alkaline potassium permanganate dropwise to this solution.
Questions :
Question 1.
Does the colour of potassium permanganate persist when it is added initially?
Answer:
When KMnO4 is added to alcohol, the colour of KMnO4 disappears initially, because, it oxidises ethanol to ethanoic acid and itself S is reduced to MnO2.
[Note: This reaction is known as Bayer’s test.]
Question 2.
Why does the colour of potassium perman-ganate not disappear when excess is added?
Answer:
KMnO4 solution when added more than required, then some of the KMnO4 remains unused and hence its colour does not disappear.
Activity 4.6 [T. B. Pg. 72]
Aim: To study the reaction of sodium metal with ethanol.
Activity:
- Teacher’s demonstration
- Drop a small piece of sodium, about the size j of a couple of grains of rice into ethanol? (absolute alcohol).
Questions:
Question 1.
What do you observe?
Answer:
Hydrogen gas is evolved due to reaction between ethanol and sodium metal.
Question 2.
How will you test the gas evolved?
Answer:
When a burning matchstick is brought near? the hydrogen gas, it burns with a popping sound.
Activity 4.7 [T. B. Pg. 73]
Aim : To study the strength of dilute acetic acid and dilute hydrochloric acid.
Activity:
Compare the pH of dilute acetic acid and dilute hydrochloric acid using both litmus paper and universal indicator.
Questions:
Question 1.
Are both acids indicated by the litmus test?
Answer:
Both acids turn blue litmus to red, thus, both the acids are indicated by the litmus test.
Question 2.
Does the universal indicator show them as equally strong acids?
Answer:
The pH values of both acids of same concentration as obtained by using universal indicator are different. The pH value of dilute acetic acid is 4, while that of dilute hydrochloric acid is 2, which indicates that acetic acid is a weak acid than hydrochloric acid.

Activity 4.8 [T. B. Pg. 73]
Aim: To study the preparation and properties of an ester.
Activity:
- Take 1 mL ethanol (absolute alcohol) and 1 mL glacial acetic acid along with a few drops of concentrated sulphuric acid in a test tube.
- Warm in a water-bath for at least five minutes as shown in figure 4.4.
- Pour into a beaker containing 20 – 50 mL of water and smell the resulting mixture.
- When the mixture of absolute alcohol and glacial acetic acid is heated in presence of few drops of concentrated sulphuric acid, it forms a sweet smelling ester.
Activity 4.9 [T. B. Pg. 74]
Aim: To show the evolution of carbon dioxide gas by the reaction of carboxylic acid with sodium carbonate or sodium hydrogencarbonate.
- Set up the apparatus as shown in Chapter 2, Activity 2.5.
- Take a spatula full of sodium carbonate in a test tube and add 2 mL of dilute ethanoic acid.
Questions:
Question 1.
What do you observe?
Answer:
When sodium carbonate is added to 2 mL dilute solution of ethanoic acid, a brisk effervescence appear due to evolution of carbon dioxide gas.
Question 2.
Pass the gas produced through freshly prepared lime water. What do you observe?
Answer:
When the evolved gas is passed through freshly prepared lime water, it turns milky due to the formation of insoluble calcium hydroxide.
Question 3.
Can the gas produced by the reaction between ethanoic acid and sodium carbonate be identified by this test?
Answer:
Yes. Carbon dioxide gas produced by the reaction can be identified by this test.
Repeat this activity with sodium hydrogen- carbonate instead of sodium carbonate.
Activity 4.10 [T. B. Pg. 74]
Aim: To study the solubility of oil in soap solution.
Activity:
- Take about 10 mL of water in two separate test tubes.
- Add a drop of oil (cooking oil) to both the test tubes and label them as A and B.
- To test tube B, add a few drops of soap solution.
- Now, shake both the test tubes vigorously s for the same period of time.
Questions:
Question 1.
Can you see the oil and water layers separately in both the test tubes immediately after you stop shaking them?
Answer:
In test tube A, two separate layers are seen, while in test tube B separate layers are not seen.
Question 2.
Leave the test tubes undisturbed for some time and observe. Does the oil layer separate out? In which test tube does this happen first?
Answer:
In test tube A, two separate layers of oil and water are formed, while, in test tube B separate layers are not formed.
Activity 4.11 [T. B. Pg. 76]
Aim : To study the reaction of soap with hard and soft water.
Activity:
- Take about 10 mL of distilled water (or rain water) and 10 mL of hard water (from a tube) well or hand pump) in separate test tubes.
- Add a couple of drops of soap solution to both.
- Shake the test tubes vigorously for an equal period of time and observe the amount of foam formed.
Questions:
Question 1.
In which test tube do you get more foam?
Answer:
The test tube which contains soft water, i.e., distilled water or rain water, produces foam easily.
Question 2.
In which test tube do you observe a curdy white precipitate?
Answer:
The test tube which contains hard water, i.e., tube well water, produces curdy white precipitates.
Note for the teacher:
If hard water is not available in your locality, prepare Some hard water by dissolving hydrogen-carbonates/sulphates, chlorides of calcium or magnesium in water.
Activity 4.12 [T. B. Pg. 76]
Aim : To study the solubility of soap and detergent in hard water.
Activity:
- Take two test tubes with about 10 mL of hard water in each.
- Add five drops of soap solution to one and five drops of detergent solution to the other.
- Shake both test tubes for the same period.
Questions:
Question 1.
Do both test tubes have the same amount of foam?
Answer:
Both the test tubes do not contain the same amount of foam. The test tube with soap solution contains little foam in it.
Question 2.
In which test tube is a curdy white solid formed?
Answer:
The test tube to which soap solution was added produced a curdy white solid substance.
![]()
![]()