JAC Board Class 9th Science Chapter 7 Notes Diversity in Living Organisms
→ Biodiversity: The word ‘biodiversity’ is used to define the diversity of life forms. Biodiversity is a word more often used to refer to the variety of life forms found in a particular geographic region.
→ Diversity: Diversity of life forms in a geographic region provides stability in that region.
→ Taxonomy: It is a branch of biology . which deals with identification, nomenclature and classification of organisms. Carolus Linnaeus is called the father of taxonomy.
→ Classification: The method of arranging organisms into groups or sets on the basis of similarities and differences is called classification.

→ Importance of Classification
a. It makes the study of a wide variety of organisms easy and systematic.
b. It helps to understand how the different organisms have evolved with time.
c. It helps to understand the inter-relationships among different groups of organisms.
d. It forms a base for the study of other biological sciences, like biogeography.
→ Classification of organisms by Aristotle: Greek philosopher, Aristotle first classified the animals based on their place of residence, i.e., whether they lived on land, in water or in the air. However, this was not an appropriate way to group organisms as animals living in the same habitat can have very different characteristics.
→ Later, all the living organisms were identified and categorised on the basis of their body structure and function. The idea of evolution was first described by Charles Darwin in 1859 in his book ‘The Origin of Species.’
→ Basis of Classification: There are certain features or properties used for the classification of living organisms which are known as characteristics. Organisms with same characteristics are placed in same groups.
→ The major characteristics considered for classification of all organisms into five major kingdoms are:
a. Type of cellular organisation
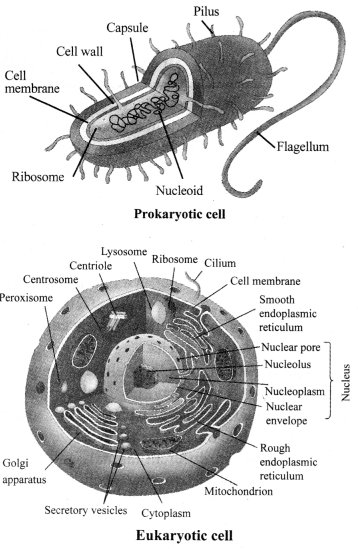
i. Prokaryotes: The organisms which have cells without well defined nucleus are called prokaryotes.
ii. Eukaryotes: The organisms which have cells with well defined nucleus are called eukaryotes. Presence of nucleus and membrane bound organelles give better efficiency to the cells.
b. Body organisations
i. Unicellular: The organisms made up of a single cell alone are termed as unicellular organisms. In them, the single cell is responsible for carrying out all the necessary functions to maintain life.
ii. Multicellular: The organisms made up of more than one cell are called multicellular organisms. Because of more number of cells, there can be some division of labour to gain more efficiency.
c. Mode of obtaining food
i. Autotrophs: Organisms producing their own food are called autotrophs. All green plants are examples of autotrophs. They have a pigment called chlorophyll, which facilitates photosynthesis.
ii. Heterotrophs: Organisms who are dependent on either plants or animals are called heterotrophs. They do not have chlorophyll. All animals, fimgi, various bacteria and protozoa belong to this group.

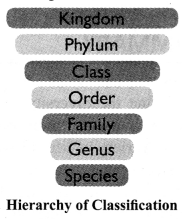
→ Hierarchy of Classification:
Linnaeus proposed a classification system that arranged organisms into taxonomic groups at different levels according to the characteristics they have. The groups or the levels from top to bottom are:
→ Nomenclature: An organism can have different names in different languages. This creates confusion in naming the organism. So, a scientific name is needed which is accepted globally. Binomial system of nomenclature given by Carolus Linnaeus is used for naming different organisms.
→ Following are some conventions in writing the scientific names:
a. Genus should be written first, followed by the name of the species.
b. First letter of the genus should be capital and that of the species should be in small letter.
c. When printed, the name should be written in italics and if hand written, the genus and the species should be underlined separately.
For example – Homo sapien for human, Panthera tigris for tiger.
→ Classification System
a. Two Kingdom Classification: Carolus Linnaeus in 1758 classified the living organisms into two groups, viz., plants and animals.
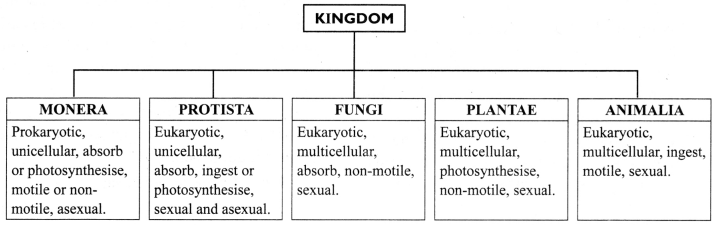
b. Five Kingdom Classification: Whittaker in 1959, further classified the organisms into five kingdoms,viz. Monera, Protista, Fungi, Plantae and Animalia.
→ Carl Woese in 1977 further divided kingdom Monera into Archaebacteria (or Archaea) and Eubacteria (or Bacteria).

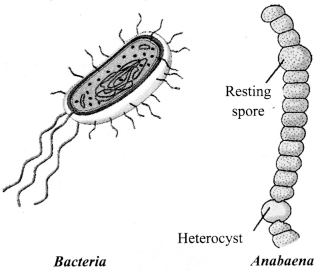
→ Kingdom Monera
a. Prokaryotic, unicellular.
b. Can be autotrophic or heterotrophic.
c. May or may not have a cell wall.
d. Examples: Anabaena and bacteria (heterotrophic), Cyanobacteria or blue green algae (autotrophic).
→ Kingdom Protista
a. Eukaryotic, unicellular.
b. Can be autotrophic or heterotrophic.
c. Have cilia, flagella or pseudopodia for locomotion.
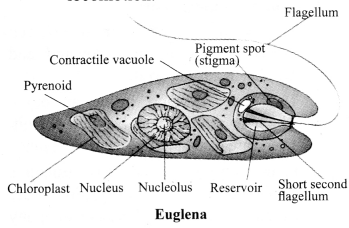
d. Examples: Plant like – unicellular algae, diatoms; Animal like – protozoans (Amoeba, Paramecium, Euglena); Fungi like – slime moulds and water moulds.
→ Kingdom Fungi
a. Heterotrophic, eukaryotic organisms.
b. Saprophytic. They use.decaying organic materials as food.
c. Some fungi live in a symbiotic relationship with cyanobacteria. They are called lichens. The algal part provides food and the fungal part provides minerals and substratum.
d. Cell wall is made up of chitin.
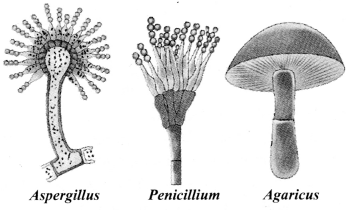
e. Examples: Mushrooms (Agaricus), green mould (Penicillium), smut (Aspergillus).
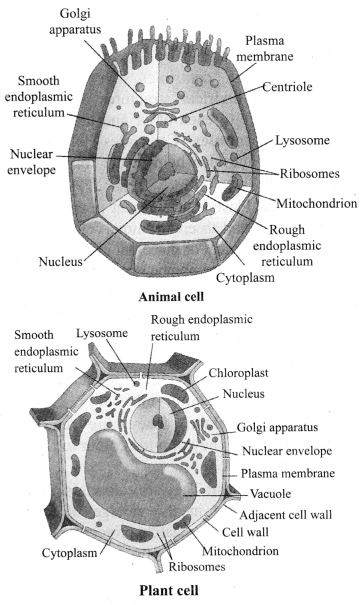
→ Kingdom Plantae:
a. Multicellular, eukaryotic.
b. Autotrophs, use chlorophyll for photosynthesis.
c. Cellulosic cell wall present.
→ Kingdom Animalia:
a. The organisms of Animalia include all organisms which are multicellular, eukaryotic and without cell wall.
b. Organisms of kingdom Animalia are heterotrophs.
→ Classification of Kingdom Plantae
Based upon body differentiation, types of vascular tissues (xylem or phloem), reproductive structures (seeds or spores) and type of seeds, (covered or naked), kingdom plantae is divided into the following divisions:
→ Division 1: Thallophyta
a. The plants of thallophyta do not have well differentiated body.
b. The plants in thallophyta are known as algae and they are predominantly aquatic.

c. Examples: Spirogyra, Ulothrix, Cladophora, Chara, etc.

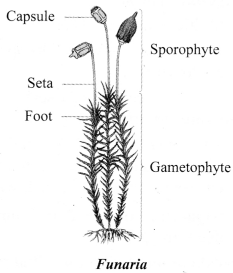
→ Division 2: Bryophyta
a. The plants of this group are called amphibians of plant kingdom.
b. Though not distinctly developed, plant body can be differentiated to form stem and leaf like-structures.
c. Examples: Moss (Funaria) and Marchantia.
→ Division 3: Pteridophyta
a. Plants of Pteridophyta have defined roots, stems and leaves.
b. These plants have specialised tissues that transport water and other materials from one part to another part of the plant.
c. Examples: Marsilea, fem and horse-tails.
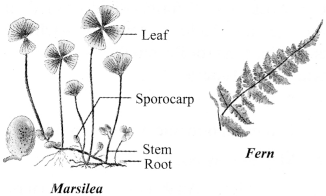
d. The similarity among the thallophytes, the bryophytes and the pteridophytes is that all of them have naked embryos, which are known as spores.
e. The plants of these groups are known as ‘cryptogams’, which means ‘hidden reproductive organs’.
→ Division 4: Gymnosperms
a. The plants of this group bear naked seeds and are usually perennial, evergreen and woody.

b. Examples: Pine, Cycas and Deodar.
→ Division 5: Angiosperms
a. This word is made from two Greek words: angio- meaning covered and sperma- meaning seed.
b. The seeds develop inside an organ which is modified to become a fruit. These are also called flowering plants.
c. Plant embryo in seeds has structures called cotyledons. Cotyledons are called ‘seed leaves’ because in many instances they emerge out and become green when the seed germinates.
d. The angiosperms are divided into two groups on the basis of the number of cotyledons present in the seed:
- Monocots (Monocotyledonous): These seeds have a single cotyledon. E.g., onion.
- Dicot (Dicotyledonous): These seeds have two cotyledons. E.g., musturd.

→ Classification of Kingdom Animalia
Based on the extent and type of the body design differentiation, Animal kingdom is classified into the following phyla:
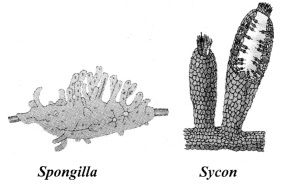
→ Phylum Porifera
a. Cellular level of organisation.
b. Non-motile animals.
c. Holes on the entire body surface which lead to a canal system for circulation of water and food.
d. Hard outside layer known as skeleton.
e. Examples: Sponges (Spongilla, Sycon).
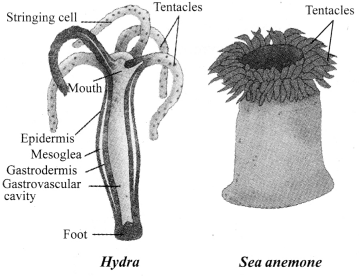
→ Phylum Coelenterata
a. Tissue level of organisation.
b. No coelom.
c. Radial symmetry’, diploblastic.
d. Hollow gut, move from one place to another.
e. Examples: Hydra, sea-anemone, jelly fish, corals.
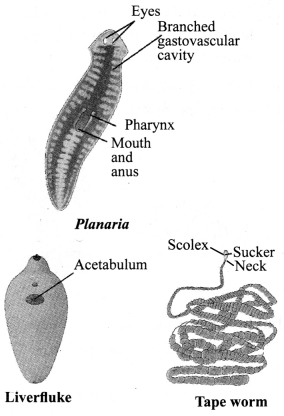
→ Phylum Platyhelminthes
a. Well-developed organs.
b. No coelom.
c. Known as flatworms.
d. Bilateral symmetry, triploblastic.
e. Free living or parasitic.
f. Digestive cavity has a single opening for ingestion and egestion.
g. Examples: Planaria, liverfluke, tape worm, etc.
→ Phylum Nematoda
a. Cylindrical body.
b. No well-developed body (i.e., no real organ).
c. False coelom.
d. Bilateral symmetry, triploblastic.
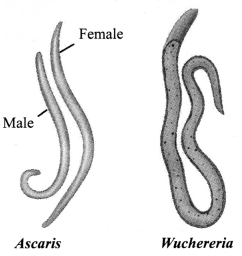
e. Many are parasitic worms living inside human body and can cause various diseases, like filarial worms cause elephantiasis, round worms and pin worms living in human intestine cause infection.
f. Examples: Ascaris, Wuchereria.

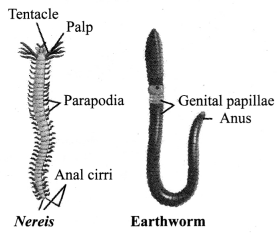
→ Phylum Annelida
a. Found everywhere including fresh water, marine water as well as on land.
b. Bilaterally symmetrical and triploblastic body.
c. True body cavity present.
d. Segmented (segments specialised for different functions).
e. Extensive organ differentiation.
f. Examples: Earthworm, Nereis, leeches.
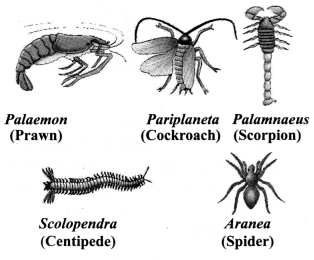
→ Phylum Arthropoda
a. The largest group of animals.
b. Open circulatory system.
c. Generally known as insects.
d. Bilateral, triploblastic.
e. Segmented, sometimes fused.
f. Jointed appendages like feet, antenna.
g. Examples: Prawns, butterflies, houseflies, spiders, scorpions, etc.
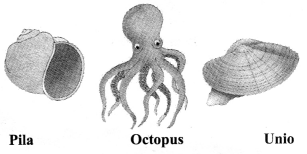
→ Phylum Mollusca
a. Coelom present.
b. Triploblastic, bilateral symmetry.
c. Soft bodies, sometimes covered with shell.
d. Generally not segmented.
e. No appendages present.
f. Kidney-like organs for excretion.
g. Examples: Chiton, Octopus, Pila, Unio.
→ Phylum Echinodermata
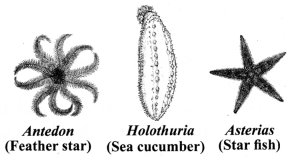
a. Spiny skinned.
b. Free-living marine organisms.
c. Triploblastic with coelom present.
d. Skeleton of calcium carbonate.
e. Water vascular system for locomotion.
f. Bilateral symmetry before birth.
g. Examples: Starfish, sea cucumber,
feather star, etc.
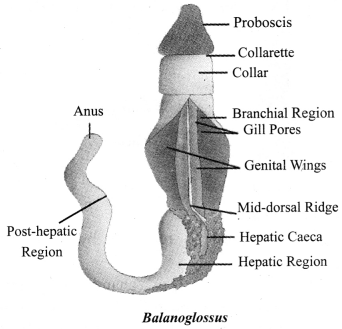
→ Phylum Hemichordata
a. Small group of marine animals.
b. Cylindrical, bilateral symmetry, triploblastic.
c. Coelom present.
d. Gills for respiration.
e. Examples: Balanoglossus.

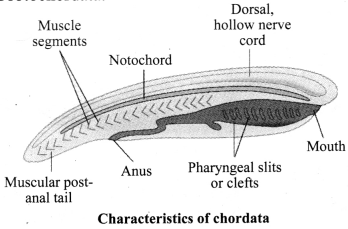
→ Phylum Chordata
a. Bilateral symmetry, triploblastic.
b. Coelom present.
c. Notochord present.
d. Gills presented at some phase of life.
e. Dorsal nerve chord.
f. Post-anal tail present at some stage of life, e.g., in human beings during embryonic stages.
Phylum chordata is further subdivided into three sub-phyla, namely, urochordata, cephalochordata and vertebrata. Urochordata and cephalochordata are together called as Protochordata.
a. Protochordata
- Bilaterally symmetrical, triploblastic.
- Coelom present.
- Marine.
- Examples: Herdmania, Amphioxus,
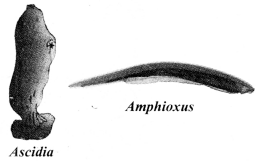
b. Vertebrata
- Notochord is replaced by vertebral column
- Two, three, four chambered heart. Examples: Human (4 chambered), frog (3 chambered), fishes (2 chambered).
- Organ for excretion, e.g., kidney, is present.
- Paired appendages.
→ Classification of Vertebrata
The organisms of this kingdom have a true vertebral column and an internal skeletal structure. Vertebrates are further classified into the following classes:
Cyclostomata:
a. Jawless vertebrates.
b. Elongated body.
c. Circular mouth, slimy skin, scaleless.
d. Ectoparasites or borers of other vorebebrates.
e. Example: Petromyzon, Myxine (Hagfish).

Pisces:
a. The organisms of this group are typically different types of fishes.
b. Fishes can live only in water.
c. The skin of fishes is covered with scales/plates.
d. Fishes use oxygen dissolved in water to breathe with the help of the gills.
e. The tail of a fish helps in its movements.
f. Fishes are cold-blooded organisms and their hearts have only two chambers.
g. Fishes lay eggs (oviparous).
h. Examples: Rohu, Catla, Scoliodon, etc.
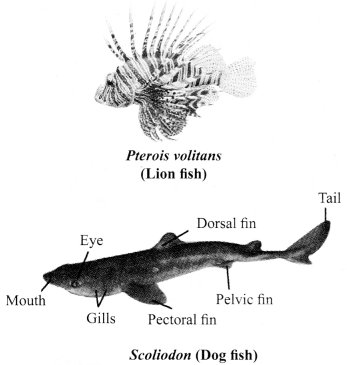

Amphibia:
a. The organisms of Amphibia have mucus glands in the skin, and they have three- chambered heart.
b. Amphibians can live in water as well as on land.
c. These organisms respire through either gills or lungs.
d. They lay eggs.
e. Examples: Salamander, toad, frog, etc.

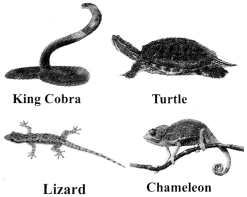
Reptilia:
a. The organisms of Reptilia are cold blooded. They cannot maintain a constant body temperature.
b. They have three-chambered heart, except crocodiles which have four- chambered heart.
c. These organisms lay eggs with tough coverings (oviparous).
d. They have scales.
e. Examples: King cobra, turtle, chameleon, lizard, etc.
Aves:
a. Aves are warm-blooded.
b. These organisms lay eggs.
c. Most of the Aves have feathers.
d. Four-chambered heart is present.
e. They have hollow bones which help them to fly.
f. Examples: Pigeon, crow, etc.

Mammalia
a. The organisms of Mammalia are warmblooded and they have four-chambered heart.
b. Mammalia are typically characterised for their mammary glands.
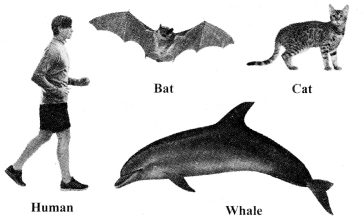
c. Mammary glands produce milk to nourish the young ones.
d. Most of the mammals give birth to young ones. However, a few mammals, such as the Platypus and the Echidna, lay eggs.
e. Skin of mammals has hair along with sweat and oil glands.
f. Examples: Human, bat, cat, whale, etc.
![]()
![]()