JAC Board Class 9th Science Chapter 2 Notes Is Matter Around Us Pure
→ A pure substance consists of a single type of particles.
→ A mixture has more than one substance (element / compound) mixed in any proportion.
→ Mixtures can be separated into pure substances by appropriate separation techniques.
→ A solution is a homogeneous mixture of two or more substances. The major component of a solution is known as the solvent and the minor one is the solute. For example, sugar dissolved in water or alloy of copper and zinc or ethyl alcohol in water.
- Mass percentage of a solution
= \(\frac{\text { Mass of solute }}{\text { Mass of solution }}\) × 100 - Volume percentage of a solution
= \(\frac{\text { Mass of solute }}{\text { Mass of solution }}\) × 100
→ Materials which are insoluble in a solvent and have particles which are visible to naked eyes form a suspension. A suspension is a heterogeneous mixture.
→ When solids are dispersed in liquids to form a heterogeneous mixture or an opaque medium, it is called suspension.
![]()
→ Colloidal solutions are heterogeneous mixtures where particles of 1 × 109 to 1 × 10-6 m diameter, called dispersed phase, are distributed uniformly in a solvent, called dispersing medium.
→ Difference between true solutions, suspension and colloidal solutions.
| True Solution | ColloidalSolution | Suspension |
| It is a homogeneous mixture of solute and solvent. | It appears to be homogeneous but actually it is a heterogeneous mixture of dispersed phase and dispersing medium. | It is a heterogeneous mixture. |
| The solute particles are very small, i.e., less than 109 m in diameter. | The solute particles are between 109 and 106 m in diameter. | The solute particles are quite large, i.e., larger than 106 m in diameter. |
| Particles of true solution are not visible to naked eye. | Particles are not visible to naked eye but can be seen with ultramicroscope. | Particles are big enough to be seen by naked eye. |
| The entire solution passes through filter paper. | The particles can pass through ordinary filter paper. | The particles cannot pass through filter paper. |
| The solute particles do not show Tyndall effect. | The particles show Tyndall effect. | The particles may or may not show Tyndall effect. |
| The particles do not settle down. | The particles do not settle down. | The particles may settle due to gravity. |
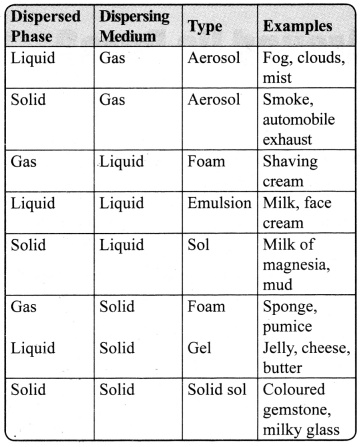
→ Different Types of Colloids
→ Separation of a mixture can be done by hand-picking, sieving, winnowing, sedimentation, decantation, filtration, evaporation, distillation, fractional distillation, centrifugation, crystallisation and chromatography.
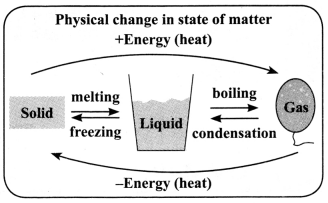
→ A physical change brings about a change in the state of matter without change in the composition or chemical nature of the substance.
→ A chemical change brings about a change in chemical properties of matter because one or more substances are transformed into a new substance.
→ Pure substances can be elements or compounds.
→ An element is a form of matter which cannot be broken down by chemical reactions into simpler substances.
![]()
→ A compound is a substance composed of two or more different types of elements, chemically combined in a fixed proportion.
→ Properties of a compound are different from its constituent elements whereas a mixture shows the properties of its constituting elements or compounds.