Jharkhand Board JAC Class 10 Science Important Questions Chapter 1 Chemical Reactions and Equations Important Questions and Answers.
JAC Board Class 10 Science Important Questions Chapter 1 Chemical Reactions and Equations
Additional Questions and Answers
Question 1.
Answer the following questions :
(1) State the type of reaction in the following :
(i) Vegetable matter changing into compost.
(ii) Burning of natural gas
(iii) Adding water to quick lime to form slaked lime.
Answer:
(i) Vegetable matter changing into compost is an exothermic and decomposition reaction.
(ii) Burning of natural gas is an exothermic reaction.
(iii) Adding water to quick lime to form slaked lime is combination reaction, which is exothermic.
(2) Identify the substance which acts as an oxidising agent and reducing agent in the following reaction :
(i) H2(g) + Cl2(g) → 2HCl(g)
(ii) 2Al(s) + Cr2O3(s) > Al2O3(s) + 2Cr(s)
Answer:
Cl2 in reaction (i) and Cr2O3 in reaction (ii), acts as an oxidising agent.
While, H2, in reaction (i) and Al, in reaction (ii) acts as a reducing agent.
![]()
(3) AgNO3(aq) + NaCl(aq) → AgCl(s) ↓ + NaNO3(aq)
FeS(s) + H2SO4(aq) → FeSO4 + H2S ↑
In above chemical equations, two different type of arrows (4- and t) are shown with products. What does these arrows represent?
Answer:
↓ Indicate the formation of insoluble ↑ substance (precipitate) while ↑ represent the evolution of gas.
(4) Distinguish between :
1. Endothermic reaction and Exothermic reaction
Answer:
| Endothermic reaction | Exothermic reaction |
| 1. A chemical reaction in which heat energy is absorbed during the formation of product is called an endothermic reaction. | 1. A chemical reaction in which heat energy is evolved during the formation of product is called an exothermic reaction. |
| 2. For example, reaction of barium hydroxide with ammonium chloride is an endothermic reaction. | 2. For example, reaction of quick lime with water is an exothermic reaction. |
2. Oxidation reaction and Reduction reaction
Answer:
| Oxidation reaction | Reduction reaction |
| 1. A chemical reaction in which substance gains oxygen or loses hydrogen is called an oxidation reaction. | 1. A chemical reaction in which substance gains hydrogen or loses oxygen is called a reduction reaction. |
| 2. For example, C + O2 → CO2 2Cu + O2 → 2CuO |
2. For example, CuO + H2 → Cu + H2O CO2 + H2 → CO + H2O |
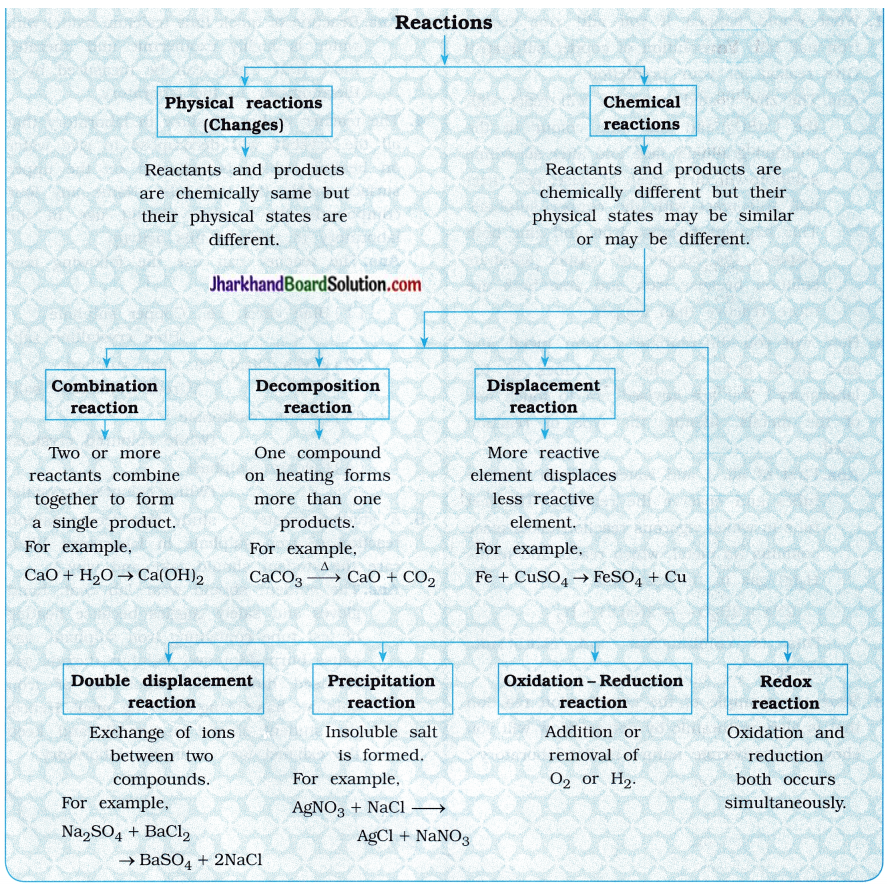
(5) What happens when carbon dioxide and water react in the same ratio?
Answer:
When six molecules of carbon dioxide and six molecules of water undergo reaction, it forms glucose with evolution of oxygen gas.
(6) How can the black coating of copper oxide be removed chemically?
Answer:
The black coating of copper oxide can be removed chemically by passing the hydrogen gas over heated copper oxide. As a result, the black coating turns brown in colour as oxygen is removed by hydrogen.
CuO + H2 → Cu + H2O
(7) When quick lime is added to water, a hissing sound is produced. Write the chemical reaction and state the type of reaction that takes place.
Answer:
CaO + H2O → Ca(OH)2 + heat
This type of reaction is exothermic and combination.
(8) Write the name of the products obtained and type of reaction given below:
Na2SO4 + BaCl2 → …………. + …………..
Answer:

(9) Reactants A and B react together and forms zinc chloride and hydrogen gas. Identify A and B. Write the chemical equation.
Answer:
The reactant A is a zinc metal and the reactant B is hydrochloric acid.
Zn + 2HCl → ZnCl2 + H2
(10) State the ascending order of reactivity for Cu, Ag and Fe metals based on the reaction given below:
(i) Fe(s) + CuSO4(aq) → FeSO4(aq) + Cu(s)
(ii) Cu(s) + FeSO4(aq) → No reaction
(iii) Cu(s) + 2AgNO3(aq) → Cu(NO3)2(aq) + 2Ag(s)
(iv) 2Ag(s) + CU(NO3)2 → No reaction
Answer:
Based on the given reaction, ascending order of reactivity of Cu, Ag and Fe is as follows :
Ag < Cu < Fe
(11) State the true and false option for the chemical reaction given below:
3Fe(s) + 4H2O(g) → Fe3O4(s) + 4H2(g)
(i) Fe is being oxidised.
(ii) Water is being reduced.
(iii) Water acts as reducing agent.
(iv) Water acts as oxidising agent.
Answer:
Here, (i), (ii) and (iv) are true options, while (iii) is a false option.
(12) Choose the true and false statements for the reaction CuO + H2 → Cu + H2O.
(i) CuO is an oxidising agent.
(ii) H2 is being oxidised.
(iii) The reaction is a displacement reaction.
(iv) The valency of Cu is not changing.
Answer:
Statements, (i), (ii) and (iii) are true, while statement (iv) is false.
![]()
(13) Three test tubes are taken and marked as ‘X’, ‘Y’ and ‘Z’. In test tube X, iron nail is dipped in water. In test tube Y, iron nail is dipped in mixture of water and oil. In test tube Z, iron nail is added with dry CaCl2. In which test tube, the iron nail will rust? Why?
Answer:
The iron nail will rust in test tube X, because it provides the essential moist conditions for rusting since both moisture as well as air is present in the test tube.
(14) A metal ‘M’ kept in air turns green and when it is heated, it turns black. Name the metal and the compound formed in both cases.
Answer:
Metal M is copper. Green compound is due to formation of copper carbonate and black coloured compound is due to formation of copper oxide.
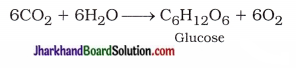
(15) State the equations of zinc and lead, where it displaces copper from its compounds.
Answer:
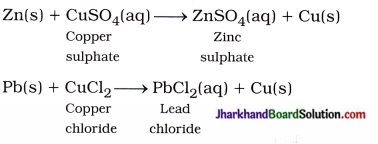
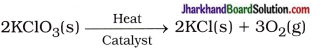
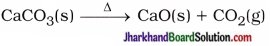
(16) Write balanced equations for thermal decomposition and photochemical decomposition reactions.
Answer:
Thermal decomposition reaction :
Photochemical decomposition reaction :
![]()
(17) Name the methods used to prevent rusting.
Answer:
- Oiling of metals
- Applying paints
- Preparation of an alloy and
- Electroplating or galvanising.
(18) Why does most of the metal articles become dull, when left in an open air?
Answer:
Metal articles left in an open air reacts with the gases of atmosphere (air) and forms a ( layer of oxide compound on their surface which make metal dull and the lustre is lost.
For example, aluminium metal reacts readily l with oxygen of air and forms a layer of aluminium oxide and the surface of Al becomes dull.
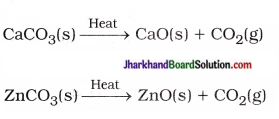
(19) Give two examples of a reaction which is both endothermic and decomposition in nature.
Answer:
(20) What is called rancidity? What is the general name of chemicals which are added to fat and oil containing food to prevent the rancidity?
Answer:
When oil and fat containing food is i exposed to air, it reacts with oxygen and gets? oxidised. As a result, food become rancid and S their taste and smell changes. This process is called rancidity.
The general name of the chemicals which are added to food to prevent its rancidity are known as antioxidants. For example, Nitrogen gas is an antioxidant substance.
(21) Why is photosynthesis considered as an endothermic reaction?
Answer:
In photosynthesis reaction, energy is required to form glucose from carbon dioxide and water. Energy, in the form of sunlight is used for s photosynthesis. Hence, this reaction is called an endothermic reaction.
(22) What is meant by electrolysis? Mention its two uses with examples.
Answer:
When electric current is passed through an aqueous solution or molten ionic compound, } the ions of the compound form products on electrodes by electrolytic oxidation – reduction. This process is called an electrolysis.
Uses:
1. Gas and metal can be obtained from an aqueous solution of salt.
For example, Electrolysis of an aqueous solution of NaCl produces Na metal and Cl2gas.
2. The coating (protective layer) of one metal can be formed on the surface of another metal.
For example, Gold plating can be carried out on the surface of silver articles.
Zn layer can be formed on the surface of iron.
(23) Write the definition of displacement reaction. Give one example of it and explain, how is it different from double displacement reaction.
Answer:
The reaction in which more reactive metal displaces the less reactive metal from its compound is called as displacement reaction.
For example,
Fe(s) + CuSO4(aq) → FeSO4(aq) + Cu(s)
This reaction is different than double displacement reaction. In double displacement reaction ions get exchanged in two compounds.
For example,
Na2SO4(aq) + BaCl2(aq) → BaSO4(s) + 2NaCl(aq)
In short, one ion gets exchanged in displacement reaction, while two ions are exchanged in double displacement reaction.
(24) How does a chemical equation makes a reaction more informative?
Answer:
For making a chemical equation more informative –
- The physical states of the reactants and products are represented by symbols (g) for gas, (l) for liquid, (s) for solid and (aq) for aqueous solution.
- Specific conditions like temperature, pressure, catalyst are shown above/below the arrow in the equation.
(25) Give the balanced chemical equations for the following :
(i) Reaction used in black and white photography
(ii) Reaction of oxidation of glucose
(iii) Formation of water from H2 and O2
Answer:
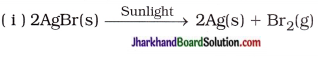
(ii) C6H12O6(s) + 6CO2(g) → 6CO2(g) + 6H2O(g) + Energy
(iii) 2H2(g) + O2(g) → 2H2O(l)
(26) What is a redox reaction? When a magnesium ribbon is burnt in air, it burns with a dazzling white flame and white powder is formed. Is magnesium oxidised or reduced? Why?
Answer:
A reaction in which oxidation and reduction reactions occur simultaneously is called redox reaction.
2Mg(s) + O2(g) → 2MgO(s)
In this reaction, magnesium combine with oxygen. Hence, it is oxidised to MgO.
![]()
(27) Name different types of chemical reactions; and explain it with suitable example.
Answer:
Name of different types of chemical reactions with suitable example are given below :
1. Combination reaction : Two or more than two reactants combine together to form a single product.
For example, CaO(s) + H2O(l) → Ca(OH)2(aq)
2. Decomposition reaction : A single reactant, on heating decomposes to form more than one product.
For example,
3. Displacement reaction : More reactive metal displaces the less reactive metal from its compound.
For example,
Fe(s) + CuSO4(aq) → FeSO4(aq) + Cu(s)
4. Double displacement reaction : In this an exchange of ions occurs between two compounds.
For example,
Na2SO4(aq) + BaCl2(aq) → BaSO4(s) + 2NaCl(aq)
5. Oxidation reaction : A reaction in which oxygen is added or hydrogen is removed is called an oxidation reaction.
For example, C(s) + O2(g) → CO2(g)
6. Reduction reaction : A reaction in which hydrogen is added or oxygen is removed is called reduction reaction.
For example, CuO + H2 → Cu + H2O
7. Precipitation reaction : In this reaction, insoluble salt is formed.
For example,

(28) (i) What is called rancidity?
(ii) Suggest two methods to reduce the effect of rancidity.
(iii) How is corrosion different from rusting?
Answer:
(i) Rancidity : Oil and fat containing food when exposed to air gets oxidised and it becomes rancid due to which its taste and smell change. This reaction is called rancidity. Such food items are not suitable for consumption.
(ii) To reduce the problem of rancidity –
- Keep the food in a closed container.
- Use antioxidants.
(iii) Corrosion is observed in all the metals, when exposed to air; and a layer of compound s is formed due to reaction of metal with moisture, acid and gases present in atmosphere.
Rusting is a process in which iron reacts with t air and moisture to form brownish red coloured powder called rust. Due to corrosion, metal lose their lustre and become dull while due to rusting iron metal is > slowly destroyed.
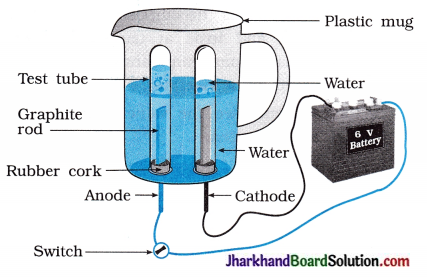
(29) Water contains hydrogen and oxygen in the ratio of 2:1. Prove it by an activity.
Answer:
Take a plastic mug. Drill two holes at its base and fit rubber stoppers into these holes. S Insert carbon electrodes in these rubber stoppers as shown in the figure.
- Connect electrodes to a 6 volt battery.
- Fill the mug with water such that the electrodes are immersed and add a few drops of dilute sulphuric acid to the water to make it conducting.
- Take two test tubes filled with water and invert them over the two carbon electrodes.
- Switch on the current and leave the apparatus undisturbed for sometime.
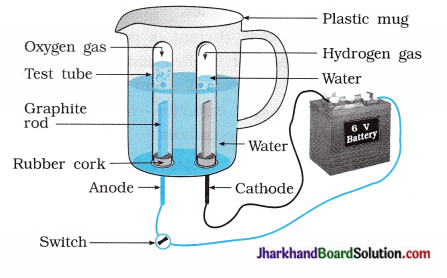
- You will observe the formation of bubbles at both the electrodes. These bubbles displace water of the test tubes.
- The gas collected in test tube attached to cathode is twice in volume than the gas collected at anode.
- Once the test tubes are filled with the gases, remove them carefully.
- The gases can be tested by bringing a burning candle close to the mouth of each test tube.
A gas at anode make the candle to light s brightly indicating it is oxygen while a gas at cathode burns with popping sound indicating it is hydrogen.
(30) What is meant by chemical equation ? Explain its characteristics with example.
Answer:
Symbolic representation of chemical reaction using symbols and formulae of the d substances involved in it is called chemical equation.
Characteristics :
1. Substances which are involved in the chemical reaction are known as reactants and S they are written on the left side of the arrow (→) sign.
2. Substances which are formed at the end of the chemical reaction are known as products s and they are written on the right side of the arrow (→) sign.
3. In chemical equation, the physical states of the reactants and products are mentioned, such as (s) for solid, (l) for liquid, (g) for gas and (aq) > for an aqueous solution. These notations (s), (1), (g) and (aq) are shown at right (just after their formula) in bracket with small letters.
For example,
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
4. In chemical equation, the gaseous product is represented by putting an arrow pointing upwards (↑) and an insoluble product (Precipitate) is represented by putting an arrow pointing downwards (↓).
For example,
- C(s) + O2(g) → CO2(g) or CO2(↑)
- AgNO3(aq) + NaCl(aq) → NaNO3(aq) + AgCl(s) or AgCl(↓)
5. Some chemical reactions take place under specific reaction conditions such as temperature, pressure, catalyst which are shown above/below the arrow in the equation.
For example,

6. According to the law of conservation of mass, chemical equation should be written as balanced equation.

Question 2.
Give scientific reasons for the following statements:
(1) Chemical equation must be balanced.
Answer:
In balanced chemical equation, the number of atoms of each element remains the same on both sides. Now, according to the law of conservation of mass, matter can neither be created nor be destroyed in a chemical reaction. Hence, the total mass of reactants and products must be equal.
As a result, it is essential to keep the number of atoms equal in both sides of chemical reaction. Thus, it is the basic need of balancing the chemical equation.
(2) Magnesium ribbon should be cleaned before burning in air.
Answer:
Magnesium ribbon is more reactive and when it is exposed to air, a layer of MgO is formed on its surface.
2Mg(s) + O2(g) → 2MgO(s)
This layer of MgO is removed by rubbing with sand paper before burning in air. Therefore, magnesium ribbon easily reacts with oxygen. Thus, magnesium ribbon is cleaned before burning in air.
(3) Lime water (solution of slaked lime) is used for whitewashing the walls of the house.
Answer:
Solution of lime water – Ca(OH)2 is used for whitewashing the walls of the house.
It is prepared by slaking of lime as shown s in the following reaction :
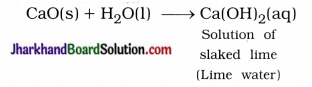
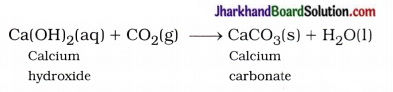
Solution of lime water is applied to the walls of the house, then it reacts slowly with the CO2 gas of air and a thin layer of CaCO3 is formed on walls after two to three days. As a result walls appear shining white.
(4) Respiration is an exothermic reaction.
Answer:
We need energy for staying alive. We get S this energy from the diet (food), we eat. Food is broken down into simple substances during digestion.
For example, Rice, potatoes and bread consists of carbohydrates. These carbohydrates are broken down to form glucose. This glucose combines with oxygen in the cells of our body and provides energy. This reaction (process) is called respiration. Thus, energy is released during respiration process, it is called an exothermic reaction.
C6H12O6(aq) + 6O2(aq) → 6CO2(aq) + 6H2O(l) + Energy
(5) Food items should be stored in air tight closed containers.
Answer:
When food items are left exposed to air, they react with oxygen and become rancid. As a result taste and smell of the food change, which is harmful to health. Thus, food items should be stored in air tight closed containers to avoid rancidity.
(6) A thin layer of zinc is applied on the plates of steamer to prevent its rusting.
Answer:
The plates of steamer consists of iron. After sometime, due to presence of water and dissolved oxygen, its surface get covered with a reddish brown flaky substance called rust.
Hence, to prevent the rusting of iron, a layer of more reactive metal Zn is applied on its surface.
Objective Questions and Answers
Question 1.
Answer the following questions in short:
(1) Write the chemical reaction taking place between lead nitrate and potassium iodide. Identify the type of reaction.
Answer:
Equation for reaction :
Pb(NO3)2(s) + 2KI(s) → PbI2(aq) + 2KNO3(aq)
It is a double displacement reaction.
(2) What is meant by skeletal equation? Give an example.
Answer:
Equation in which chemical formulae of the substances are represented briefly is called skeletal equation.
In skeletal equation, the reaction is not balanced and the mass is not the same on both sides of equation.
For example, Mg + O2 → MgO
(3 ) To which sides of a chemical equation the reactants and products are represented?
Answer:
In chemical equation, reactants are written on left side of an arrow (→), while products are written on right side of an arrow (→).
(4 ) Write the name and molecular formula of the substance used in whitewashing the walls of the house.
Answer:
Calcium hydroxide is used for white washing the walls of the house. Its molecular formula is Ca(OH)2.
(5) Blue crystals of copper sulphate on heating in a dry test tube become colourless. Why?
Answer:
The blue colour of copper sulphate is due to five water molecules attached with it. On heating, it loses water molecules; and become colourless. [It is converted into anhydrous white CuSO4.]
(6) Why does the solution of AgNOa stored in dark brown coloured bottle?
Answer:
The solution of AgNOs is decomposed in presence of sunlight hence it is stored in dark brown coloured bottle; which prevent the passage of light through the solution.
(7) What is meant by reversible reaction?
Answer:
The reaction in which the reactants have a tendency to form products while the products have a tendency to reform the reactants is called a reversible reaction.
(8) State the law of conservation of energy.
Answer:
Matter can neither be created nor be destroyed, (or Energy can neither be created nor be destroyed but can be converted from one form to another).
![]()
(9) Which carbohydrate substances, on decomposition form glucose?
Answer:
Carbohydrates such as rice, potatoes and bread, etc. form glucose on their decomposition.
(10) Which substance is reduced in the following reaction?
Reaction : 8Al + 3Fe3O4 → 4Al2O3 + 9Fe
Answer:
In this reaction, oxygen is removed from Fe3O4. Hence Fe3O4 is reduced.
(11) Which compound is used in black and white photography?
Answer:
Silver bromide or silver chloride is used in black and white photography.
(12) What characteristics of food containing oil and fat change when exposed to air for a long time?
Answer:
When food containing oil and fat are exposed to air for a long time then its taste and smell get changed due to rancidity.
Question 2.
Fill in the blanks :
- The colour of lead nitrate powder is …………….
- Respiration is an ……………. type of reaction.
- Matter can neither be created, nor be destroyed. It is called the law of …………….
- Quick lime is also known as …………….
- Burning of coal and formation of water is ……………. type of reaction.
- During electrolysis of water, electrodes are connected with ……………. V of battery.
- When iron nail is dipped in a solution of copper sulphate, then, the colour of the solution becomes ……………. after sometime.
- Reaction,

is a ……………. reaction. - ……………. is used in black and white photography.
- Reducing agent undergoes …………….
- Pb(s) + ……………. → PbCl2(aq) + Cu(s)
- ……………. is a more reactive metal among Fe and Mg.
Answer:
- white
- exothermic
- conservation of mass
- Ca(OH)2 or Calcium hydroxide
- combination
- 6
- Pale green
- decomposition
- AgBr or AgCl
- oxidation
- CuCl2
- Mg
Question 3.
State whether the following statements are true or false:
- Hydrogen gas was discovered by Henry Cavendish.
- (a) PbS(s) + (b) O2(g) (c) PbO(s) + (d) SO2(g).
In this reaction, the values of co-efficient (a), (b), (c) and (d), in balanced equation are taken 2, 3, 2, 2 respectively. - Oxidising agent undergoes oxidation in the reaction.
- Reducing agent gives H2 or receives O2.
- In reaction, MnO42- → MnOa + MnO41- MnO42- acts both as oxidising agent as well as reducing agent.
- Fe acts as an oxidising agent in the following reaction :
Fe + CuSO4 → FeSO4 + Cu - Heating of calcium carbonate is an endothermic reaction.
- In the reaction, 3Mg + N2 → Mg3N2, Mg undergo oxidation.
- Oxidation and reduction does not occur in redox reaction.
- In decomposition reaction, single reactant forms two or more products.
- Precipitate obtained in precipitation reaction is soluble in water.
- In rancidity, taste and smell of food item changes.
- The colour of KI is orange-grey.
- Reaction of zinc metal with dilute HCl liberates H2 gas.
- Mg is highly inactive metal.
- Ca(OH)2 is also known as lime water.
- Burning of natural gas is an exothermic reaction.
- The proportion by volume of H2 and O2 gases obtained at electrodes during electrolysis of water is 1 : 1.
- Reaction, Na2SO4 + BaCl2 → BaSO4 + 2NaCl is an example of double displacement reaction.
- MgO + H2 → Mg + H2O is an example of oxidation reaction.
Answer:
- True
- True
- False
- True
- True
- False
- True
- True
- False
- True
- False
- True
- False
- True
- False
- True
- True
- False
- True
- False
Question 4.
(A) Choose the correct option from those given below each question:
1. 6g of hydrogen is burnt in the presence of excess oxygen. The mass of water formed is :
A. 54 g
B. 108 g
C. 36g
D. 18 g
Answer:
54 g
Hint:
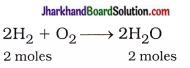
6 gm = 3 moles
From equation,
2 moles H2 = 2 moles H2O
∴ 3 moles H2 = 3 moles H2O
Now, Mass of H2O = mole x Molecular wt.
= 3 x 18 = 54g
2. Which information is not obtained by the balanced chemical equation?
A. Physical states of reactants and products.
B. Symbols and formulae of all the substances involved in a particular reaction.
C. Number of atoms/molecules of the reactants and products formed.
D. Whether a particular reaction is actually feasible or not.
Answer:
D. Whether a particular reaction is actually feasible or not.
3. Which of the following are exothermic reactions?
(i) Evaporation of water
(ii) Dilution of H2SO4
(iii) Reaction of water with quick lime
(iv) Sublimation of crystalline camphor
A. (i) and (ii)
B. (iii) and (iv)
C. (i) and (iv)
D. (ii) and (iii)
Answer:
D. (ii) and (iii)
4. Reaction : 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g) is an example of:
(i) displacement reaction
(ii) combustion reaction
(iii) redox reaction
(iv) neutralisation reaction
A. (i) and (iv)
B. (ii) and (iii)
C. (iii) and (iv)
D. (i) and (ii)
Answer:
B. (ii) and (iii)
5. Which of the following reaction occurs in whitewashing of walls?
A. 2Ca + O2 → 2CaO
B. CaO + H2O → Ca(OH)2 + heat
C. Ca(OH)2 + CO2 → CaCO3 + H2O
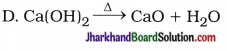
Answer:
C. Ca(OH)2 + CO2 → CaCO3 + H2O
6. What happens when crystals of lead nitrate are heated strongly in a dry test tube?
A. Crystals melt immediately.
B. Brown fumes are obtained.
C. White fumes get formed in the test tube.
D. Yellow precipitates are obtained.
Answer:
B. Brown fumes are obtained.
7. Dilute hydrochloric acid is added to test tube containing pieces of zinc. The following observations are recorded. Identify the correct observation.
A. The surface of metal becomes lustrous.
B. The reaction mixture becomes milky.
C. Odour of pungent smelling gas is experienced.
D. A colourless and odourless gas is formed.
Answer:
D. A colourless and odourless gas is formed.
8. Rancidity can be prevented by…
A. adding antioxidants.
B. storing the food in freeze.
C. keeping food away from the light.
D. All of the given.
Answer:
D. All of the given.
![]()
9. Which of the following is not a single displacement reaction?
A. CuO + H2 → H2O + Cu
B. Zn + CuSO4 → ZnSO4 + Cu
C. 4NH3 + 5O2 → 4NO + 6H2O
D. Zn + 2HCl → ZnCl2 + H2
Answer:
C. 4NH3 + 5O2 → 4NO + 6H2O
10. An element X on exposure to moist air turns reddish brown and new compound Y is formed. Identify X and Y.
A. X = Fe, Y = Fe2O3
B. X = Ag, Y = Ag2S
C. X = Cu, Y = CuO
D. X = Al, Y = Al2O3
Answer:
A. X = Fe, Y = Fe2O3
11. Identify the reducing agent in the following reaction :
Reaction : 3O2(g) + 2H2S(g) → 2H2O(l) + 2SO2(g).
A. O2
B. H2S
C. H2O
D. SO2
Answer:
B. H2S
12. Both H2 and CO2 gases are …
A. heavier than air.
B. colourless.
C. acidic in nature.
D. soluable in water.
Answer:
B. colourless.
13. Decomposition of lead (II) nitrate forms lead (II) oxide, nitrogen dioxide and oxygen gas. What is the value of co-efficient of nitrogen dioxide in the balanced equation?
A. 1
B. 2
C. 3
D. 4
Answer:
D. 4
14. When reddish brown copper metal is heated, it forms a black solid surface. Which of the following statement is incorrect?
A. Black solid substance is CuO.
B. It is redox reaction.
C. It is precipitation reaction.
D. Copper undergo oxidation.
Answer:
C. It is precipitation reaction.
15. Silver chloride is stored in dark coloured bottle because …
A. it is a white solid.
B. it gives redox reaction.
C. to avoid the effect of sunlight.
D. None of these
Answer:
C. to avoid the effect of sunlight.
16. On immersing the Zn rod in the solution of copper sulphate, you will observe …
A. deposition of Cu on Zn.
B. deposition of Zn on Cu.
C. Cu2+ oxidises.
D. blue coloured solution become more dark.
Answer:
B. deposition of Zn on Cu.
17. The reaction of H2 gas with oxygen gas forms water. This reaction is an example of:
A. combination reaction
B. redox reaction
C. an exothermic reaction
D. all of these reactions
Answer:
D. all of these reactions
18. For the reaction, CuO + H2 → Cu + H2O, choose the correct statement.
A. CuO is an oxidising agent.
B. H2 undergo oxidation.
C. It is a displacement reaction.
D. All of the given
Answer:
D. All of the given
19. Select the proper option for the following statements :
Statement 1 : Burning of magnesium ribbon in air is a redox reaction.
Statement 2 : Oxidation number of oxygen in its metal oxide is -1.
A. Statement 1 is correct.
B. Statement 2 is incorrect.
C. Statement 1 is correct, but statement 2 is incorrect.
D. Statement 1 and statement 2 both are incorrect.
Answer:
C. Statement 1 is correct, but statement 2 is incorrect.
(B) Choose more than one correct options from those given below each question:
1. Identify the type of reaction for
2Al + Cr2O3 → Al2O3 + 2Cr
(i) Oxidation
(ii) Reduction
(iii) Redox
A. Only (i)
B. Only (ii)
C. (i), (ii), (iii)
D. None of these
2. What is correct for redox reaction?
(i) Reducing agent undergoes oxidation.
(ii) Reducing agent undergoes reduction.
(iii) Reduction reaction always occurs at cathode.
(iv) Oxidising agent undergo oxidation.
A. Only (i), (ii)
B. Only (i), (iii)
C. Only (iii)
D. Given all
3. Quick lime when added in water produces hissing sound. State the type of reaction.
(i) Combination
(ii) Endothermic
(iii) Exothermic
(iv) Redox
A. Only (i)
B. Only (iii)
C. Only (i), (ii), (iii)
D. Only (iv)
Answer:
1. (i), (ii), (iii)
2. Only (i), (iii)
3. Only (i), (ii), (iii)
Question 5.
Answer the following questions in one word:
- State the formula of lime.
- Write the formula of rust.
- Name the reaction observed during rancidity of food.
- Write the formula of lead sulphate.
- What is obtained by the combustion of methane?
- Mention the respective proportion by volume of H2(g) and O2(g) obtained during electrolysis of water.
- Which coloured ash is obtained by burning 5 magnesium ribbon in air?
- What is called the insoluble substance formed during the chemical reaction?
- Name two metals which do not corrode, s
- Which gas burns with popping sound?
- Name the product formed, when silver bromide is exposed to sunlight.
- Which compound is used to detect the formation of carbon dioxide gas?
- Name the gas evolved when lead nitrate is 5 heated.
- Write the example of decomposition reaction which occurs in nature.
- Write the name of chemical used in black and white photography. I
- Which ions are present in barium sulphate?
- Mention one use of quick lime.
- Give one example of decomposition reaction in which solid and gas are formed as products.
- Give an example of antioxidant.
- Write the reaction in which hydrogen acts as a reducing agent.
- Name the reaction in which two compounds exchange their ions to form two new compounds.
- What would be the effect on lime water, when carbon dioxide gas is passed through it?
- State the molecular formula and chemical S name of iron (III) oxide.
- State different forms of energy required for breaking down the molecules of reactant in decomposition reaction.
- What does (II) indicate in iron (II) oxide?
- Give two examples of noble metals.
- Mention two examples of exothermic reactions.
- When does the breaking and forming of bonds of chemical substances take place?
- Name the reaction which forms insoluble salts.
- Write the formula for two oxides of sulphur.
- Identify the type of reaction for the following reaction :
- On immersing an iron nail in CuSO4 solution for few minutes, what will you observe?
Answer:
- CaO
- Fe2O3.XH2O
- Oxidation
- PbSO4
- CO2 and H2O
- 2 : 1
- White
- Precipitate
- Gold (Au) and Platinum (Pt)
- Hydrogen gas (H2(g))
- Silver and Bromine
- Calcium hydroxide (Ca(OH)2)
- Nitrogen dioxide and Oxygen gas
- Rottening of fruits and vegetables
- Silver bromide
- Ba2+ and SO42-
- In manufacture of cement
- Nitrogen gas
- CuO + H2 → Cu + H2O
- Double displacement reaction
- Lime water turns milky
- Molecular formula : Fe2O3 Chemical name : Ferric Oxide
- Heat, light and electricity
- Valency of Fe (Oxidation number)
- Au and Pt
- Respiration and Water added to lime
- In chemical reaction
- Precipitation reaction
- SO2 and SO3
- Decomposition and Endothermic
- Solution turns light green
Question 6.
Match the following:
(1)
| Column I (Type) | Column II (Reactions) |
| 1. Neutralisation reaction | a. Zn + Cu2+ → Zn2+ + Cu |
| 2. Redox reaction | b. CaCO3 → CaO + CO2 |
| c. HCl + NaOH → NaCl + H2O |
Answer:
(1 – c), (2 – a).
(2)
| Column I (Type) | Column II (Molecular Formula) |
| 1. Quick lime | a. CaCO3 |
| 2. Slaked lime | b. CaO |
| c. Ca(OH2) |
Answer:
(1 – b), (2 – c).
(3)
| Column I (Colour) | Column II (substance) |
| 1. Green | a. Copper sulphate |
| 2. Blue | b. Barium sulphate |
| c. Ferrous sulphate |
Answer:
(1 – c), (2 – a).
(4)
| Column I (Reaction) | Column II (Type) |
| 1. CaO + H2O → Ca(OH)2 + heat | a. Displacement |
| 2. Zn + CuSO4 → ZnSO24 + Cu | b. Double displacement |
Answer:
(1 – c), (2 – a).
Question 7.
Complete the following chemical reactions:
(1) Fe + CuSO4 → ……………… + ………………
(2) K2SO4 + BaBr2 → ……………… + ………………
(3) Pb(NO3)2 + 2KI → ……………… + ………………
(4) Zn + 2HCl → ……………… + ………………
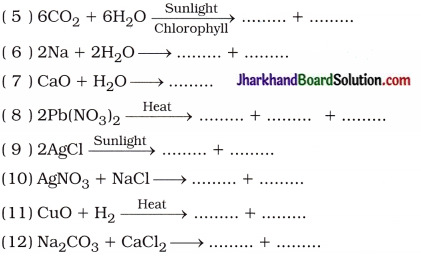
(13) Fe2O3 + 2Al > + → ……………… + ………………
(14) 2PbO + C → ……………… + ………………
(15) 2HNO3 + Ca(OH)2 → ……………… + ………………
Answer:
(1) FeSO4 + Cu
(2) 2KBr + BaSO4
(3) Pbl2 + 2KNOs
(4) ZnCl2 + H2
(5) C6H12O6 + 6O2
(6) 2NaOH + H2
(7) Ca(OH)2
(8) 2PbO + 4NO2 + O2
(9) 2Ag + Cl2
(10) AgCl + NaNO3
(11) Cu + H2O
(12) CaCO3 + 2NaCl
(13) Al2O3 + 2Fe
(14) 2Pb + CO2
(15) Ca(NO3)2 + H2O
Question 8.
Carefully observe the given diagram and answer the questions realated with it:
(1) Which gas is collected in a test tube at anode as shown in figure?
Answer:
Oxygen gas
(2) Does the reaction occur in the test tube as shown in the figure? State the change in colour.
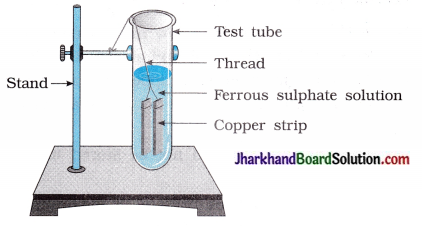
Answer:
Reaction does not occur.
Colour change does not take place.
Colour of the solution remains green.
(3) Take a small amount of ammonium chloride in a beaker as shown in figure. Add water to it. What change do you observe in temperature? Which type of reaction is this?
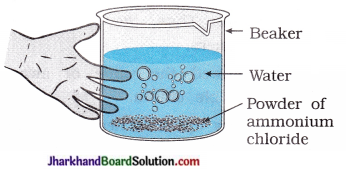
Answer:
Temperature decreases. Reaction is an endothermic.
(4) As shown in figure, the strong smell of which gas will the student experience?
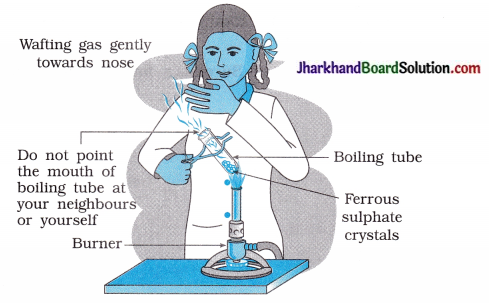
Answer:
SO2 Sulphur dioxide.
Value Based Questions With Answers
Question 1.
Hitarthi saw her grandmother storing pickles in ceramic pots. She also knew that pickles should not be stored in metal containers. Her friend often brought pickles wrapped in aluminium foil. Hitarthi advised her not to wrap pickles in foil.
- Why pickles should not be stored in metal containers?
- Which substances present in pickles reacts with the metal containers?
- What value of Hitarthi is seen in the above act?
Answer:
- Pickles reacts with the metals like copper and aluminium, hence, it should not be stored in metal containers.
- Pickles are sour in taste and contains acidic nature. Thus, they react with the metal containers.
- Hitarthi showed the value of awareness and responsible citizen.
Question 2.
Surekha was very upset as her silver jewellary had turned black; and lost its lustre. Her : father is a science teacher and he washed and cleaned the jewellary using toothpaste and s brought the shine back.
- Why does the silver jewellary tarnishes when left open?
- How had the toothpaste got the shine of silver back?
- What value of Surekha’s father is seen in this act?
Answer:
- Silver jewellary reacts with gases and moisture of air due to which it tarnishes and get corroded.
- Toothpaste reacts with black coating of silver sulphide and removes it.
- Surekha’s father showed the value of responsible behaviour and teaching art.
![]()
Question 3.
Rakesh visited the government hospital to meet his cousin. He observed the medicine in dark bottles were not stored properly. They were not kept away from light and heat. Rakesh immediately reported the same to the medical superintendent and made sure that all medicines are stored properly.
- Why are some medicines stored at cool places in dark bottles?
- Why do some medicines are kept in refrigerator?
- What value of Rakesh is seen in this act?
Answer:
- Some medicines when exposed to sunlight and high temperature, their composition changes completely. Thus, to prevent the change in composition, they are stored at cool places in dark bottle.
- Some medicines decompose and become dangerous and toxic at temperature little higher than room temperature. Hence, they are stored in refrigerator.
- Rakesh expressed the value of responsible and aware citizen.
Practical Skill Based Questions With Answers
Question 1.
What would happen if you add zinc coated iron nail into the solution of copper sulphate?
Give reason for your prediction.
Answer:
The zinc coated iron nail will react with the CuSO4 and the blue colour of the S solution gradually fade and after sometime solution will turn green because, now, iron nail will react with the copper sulphate, This happens because iron and zinc both displace the copper of copper sulphate solution because iron and zinc both are s more reactive than copper.
Question 2.
How will you differentiate sodium metal and zinc metal given in the test tubes in the laboratory? You are advised not to touch any s of the metals. Identify the type of reaction? used.
Answer:
First of all, I add water in both the test t tubes and observe the reaction. The test tube in which vigorous reaction takes place s contains Na metal, which reacts and forms H2(g) gas in the test tube.
2Na + 2H2O → 2NaOH + H2
This is combination and exothermic reaction.
Question 3.
State the example of the combination reaction > that is also exothermic by nature. How will you s show its exothermic nature in the laboratory?
Answer:
Reaction of quick lime (calcium oxide) with water is highly exothermic and liberates more heat which can be measured by a thermometer in the laboratory.
Question 4.
The teacher wishes to show in laboratory that all the crystals will decompose to give water on heating, which is collected on the upper surface inside the test tube. Name any four compounds the teacher should use in the laboratory to perform this activity.
Answer:
The teacher can use the following four compounds :
- Blue vitrol, i.e., Copper sulphate (Blue crystalline salt)
- Green vitrol, i.e., Iron sulphate (Green crystalline salt)
- Sodium carbonate (White coloured crystals)
- Calcium sulphate (White coloured crystals)
Question 5.
A student wants to study the decomposition reaction of iron sulphate in laboratory. What care the student should take and why?
Answer:
The student should wear lab coat, hand gloves and safety goggles because heating of test tube containing iron sulphate may cause burn; Moreover sulphur dioxide gas released during decomposition of iron sulphate has strong chocking smell. Hence, the student should use mask and keep the exhaust fan on in the laboratory.
Memory Map: