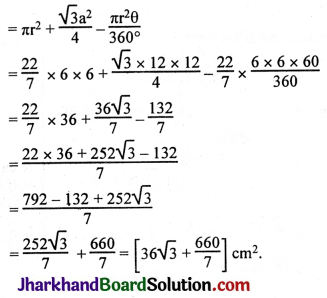
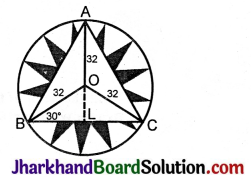
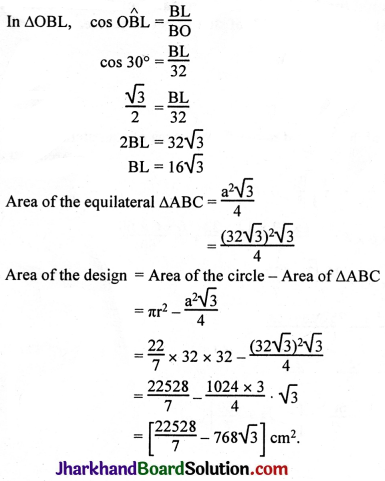
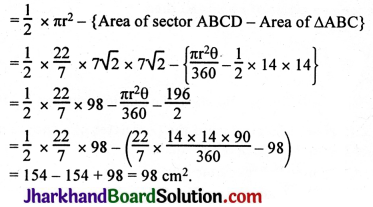
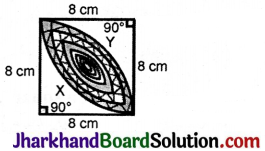
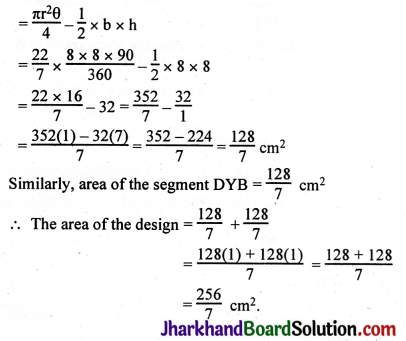
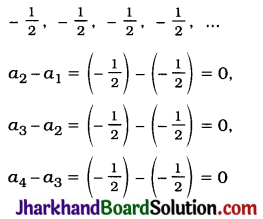
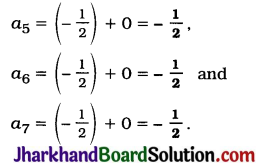
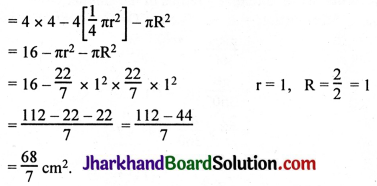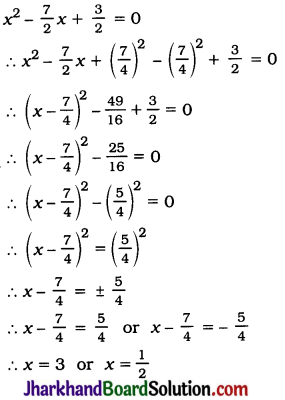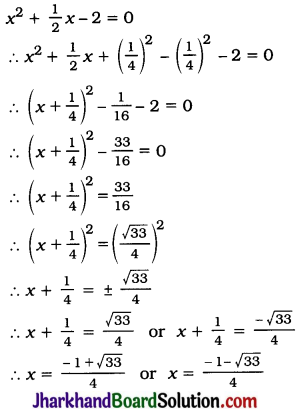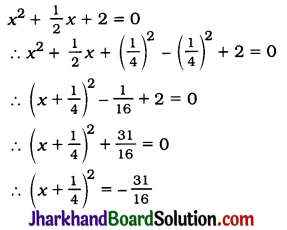JAC Board Class 9th Science Important Questions Chapter 1 Matter in Our Surroundings
Multiple Choice Questions
Question 1.
Evaporation of a liquid occurs at
(a) boiling point
(b) a fixed temperature
(c) temperature lower than boiling point
(d) all temperatures
Answer:
(c) temperature lower than boiling point
Question 2.
Intermolecular force of attraction is maximum in
(a) Solids
(b) Liquids
(c) Gases
(d) Plasma particles
Answer:
(a) Solids

Question 3.
………… will not exhibit diffusion.
(a) Hydrogen and oxygen
(b) Oxygen and water
(c) Salt and sand
(d) Sugar crystal and water
Answer:
(c) Salt and sand
Question 4.
Which of the following substances is not a solid?
(a) Air
(b) Butter
(c) Sponge
(d) Rubber band
Answer:
(a) Air
Question 5.
Which one of the following properties is not a characteristic of liquids?
(a) Fluidity
(b) Definite shape
(c) Definite volume
(d) Compressibility
Answer:
(b) Definite shape
Question 6.
Fusion is the process in which
(a) liquid changes into gas
(b) solid changes into liquid
(c) solid changes into gas
(d) gas changes into solid
Answer:
(b) solid changes into liquid
Question 7.
The density of water is maximum at
(a) 0°C
(b) 100°C
(c) 4°C
(d) 273K
Answer:
(c) 4°C
Question 8.
Choose the correct statement from the following:
(a) Two gases cannot diffuse into each other.
(b) The volume of gas expands on heating.
(c) Conversion of a gas into solid is called condensation.
(d) Gases cannot diffuse in solids.
Answer:
(b) The volume of gas expands on heating.

Question 9.
Which among the following can exist in vapour state?
(a) Oxygen
(b) Hydrogen
(c) Carbon dioxide
(d) Water
Answer:
(d) Water
Question 10.
Cooking of rice at higher altitudes is difficult because
(a) water boils at temperature <100°C
(b) water boils at 100°C
(c) boiling point of water is constant
(d) none of the above
Answer:
(a) water boils at temperature <100°C
Question 11.
At normal pressure, the boiling point of water is
(a) 98°C
(b) 100°C
(c) 110°C
(d) 90°C
Answer:
(b) 100°C
Question 12.
Gases do not have
(a) high compressibility
(b) high fluidity
(c) high density
(d) volume
Answer:
(c) high density
Question 13.
Molecules of a liquid
(a) have very strong intermolecular forces
(b) cannot move randomly
(c) have definite shape
(d) have large intermolecular spaces
Answer:
(d) have large intermolecular spaces
Question 14.
………….. decreases the rate of evaporation.
(a) Surface area
(b) Humidity
(c) Temperature
(d) Wind
Answer:
(b) Humidity
Question 15.
…………… does not convert a liquid into vapours.
(a) Boiling
(b) Evaporation
(c) Heating
(d) Condensation
Answer:
(d) Condensation
Analysing & Evaluating Questions
Question 16.
Seema visited a Natural Gas Compressing Unit and found that the gas can be liquefied under specific conditions of temperature and pressure. While sharing her experience with friends, she got confused. Help her to identify the correct set of conditions for liquefaction of gases.
(a) Low temperature, low pressure
(b) High temperature, low pressure
(c) Low temperature, high pressure
(d) High temperature, high pressure
Answer:
(c) Low temperature, high pressure
Question 17.
Under which of the following conditions, the distance between the molecules of hydrogen gas would increase?
I. Increasing pressure on hydrogen contained in a closed container.
II. Some hydrogen gas leaking out of the container.
III. Increasing the volume of the container of hydrogen gas.
IV. Adding more hydrogen gas to the container without increasing the volume of the container.
(a) I and III
(b) I and IV
(c) II and III
(d) II and IV
Answer:
(c) II and III
Question 18.
A substance has a definite shape, fixed volume, distinct boundary and cannot be compressed. What is the physical state of this substance?
(a) Solid
(b) Liquid
(c) Gas
(d) Plasma
Answer:
(a) Solid
Assertion – Reason Questions
Directions: In the following questions, the Assertions and the Reasons have been put forward. Read the statements carefully and choose the correct alternative from the following:
(A) Both the assertion and the reason are correct and the reason is the correct explanation of the assertion.
(B) The assertion and the reason are correct but the reason is not the correct explanation of the assertion.
(C) The assertion is true but the reason is false.
(D) Both statements are false.
1. Assertion: Gases diffuse faster than liquids.
Reason: Particles of gases have higher kinetic energy than those of liquids.
Answer:
(A) Both the assertion and the reason are correct and the reason is the correct explanation of the assertion.
2. Assertion: Solids have negligible compressibility.
Reason: In solids, the intermolecular force of attraction is very strong.
Answer:
(B) The assertion and the reason are correct but the reason is not the correct explanation of the assertion.
3. Assertion: Evaporation always takes place at the surface of a liquid.
Reason: Molecules at the surface of liquids have higher temperature.
Answer:
(C) The assertion is true but the reason is false.
4. Assertion: On heating or cooling, the physical state of a substance gets changed.
Reason: State of matter can be changed only by changing its temperature.
Answer:
(C) The assertion is true but the reason is false.
5. Assertion: Solids generally lack the property of diffusion.
Reason: In solids, intermolecular space is negligible.
Answer:
(A) Both the assertion and the reason are correct and the reason is the correct explanation of the assertion.
Very Short Answer Type Questions
Question 1.
What is matter?
Answer:
Anything that has mass and occupies space is called matter. For examples, rocks, plants, paper. chalk, water, food. etc.
Question 2.
In what ways are all substances around us alike?
Answer:
All the substances are matter, i.e., they all have mass and occupy space. Therefore, they are all alike.
Question 3.
Why is the rate of diffusion faster in gases?
Answer:
Because the intermolecular force of attraction is minimum in gases.

Question 4.
Why are light and sound not considered as matter?
Answer:
Light and sound are not considered as matter because they have no mass and they do not occupy space.
Question 5.
Name the three states of matter.
Answer:
Solid, liquid and gas are the three states of matter.
Question 6.
Ice and water are basically the same substance. Mention two differences in their properties.
Answer:
- Ice: It has fixed volume and definite shape. It can be stored without a container.
- Water: It is a liquid and has no definite shape. It cannot be stored without a container.
Question 7.
Classify the following materials according to the state in which they exist around us. Steel, blood, air, oil, rubber, honey, carbon dioxide, kerosene, LPG, CNG, nitrogen, oxygen
Answer:
- Solid: Steel, rubber.
- Liquid: Blood, oil, honey, kerosene, LPG.
- Gas: Air, carbon dioxide. CNG. nitrogen. oxygen.
Question 8.
Defineeflne boiling point.
Answer:
Boiling point ofa liquid is the temperature at which its pressure becomes equal to that of the atmospheric pressure and it starts vaporising into gaseous state.
Question 9.
Define melting point.
Answer:
The temperature at which a solid starts melting to become a liquid at atmospheric pressure is known as its melting point.
Question 10.
Latent heat of vaporisation of two liquids A and B is 100 kg and 150 kg respectively. What does It indicate?
Answer:
Latent heat of vaporisation depends on the nature of the liquid. Intermolecular
Question 11.
Which of the following shows the phenomenon of sublimation? Solid water, solid carbon dioxide, solid alcohol, solid oxygen
Answer:
Solid carbon dioxide.

Question 12.
How does spreading of wet clothes quicken their drying? Explain.
Answer:
Spreading of wet clothes increases surface area and so evaporation becomes faster. Thus, drying of wet clothes becomes faster by spreading them.
Question 13.
How does temperature affect the rate of evaporation?
Answer:
With increase of temperature, more number of particles get enough kinetic energy to change into the vapour state. Hence, rate of evaporation increases with increase in temperature.
Question 14.
Which will have more impact on kinetic energy: doubling mass or velocity?
Answer:
Doubling of velocity will increase kinetic energy four times. (KE = \(\frac{1}{2}\) mv2)
Question 15.
What is dry ice?
Answer:
Solid carbon dioxide obtained by cooling and applying pressure on carbon dioxide gas is called dry ice. it does not melt but directly transforms into vapour state, so it is called dry ice.
Question 16.
What is humidity?
Answer:
The air which holds water vapour is called humid air and the amount of water vapour present in the air is called humidity.

Question 17.
Define atmospheric pressure.
Answer:
The pressure exerted by the earth’s atmosphere at any given point is known as atmospheric pressure. The atmospheric pressure at sea level is atmosphere and is taken as the normal atmospheric pressure.
Analysing & Evaluating Questions
Question 18.
Alka was making tea in a kettle. Suddenly, she felt intense heat from the puff of steam gushing out of the spout of the kettle. She wondered whether the temperature of the steam was higher than that of the water boiling in the kettle. Comment.
Answer:
Temperature of steam is the same as that of boiling water. Intense heat of the steam is due to its high latent heat.
Question 19.
The energy changes in the following transformation are as follows:
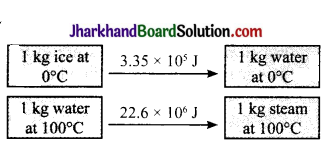
In which of the above, the intennolecu lar forces are stronger?
Answer:
The intermolecular forces are stronger in water at 100°C.
Question 20.
You are given the following substances with their melting and boiling points.
| Substance | Melting Boiling point (°C) | point (°C) |
| X | 10 | 25 |
| Y | 50 | 90 |
| Z | 15 | 80 |
Identify the physical states of X,Y and Z t the temperature of 30°C.
Answer:
‘X’ is gas at the given temperatLire.
‘Y’ is solid at the given temperature.
‘Z’ is liquid at the given temperature.
Short Answer Type Questions
Question 1.
Common salt and sugar hase similar appearance. Why are these classified as different substances?
Answer:
The substances are not classified only by their appearance. They are classified by their properties. such as density boiling point or melting point, conductivity ther mal capacit’ and other physical and chem ical properties. Common salt and sugar have diflì.rent physical and chemical prop erties and so are different substances.
Question 2.
Why do we sec water droplets collected on the outer surface of a glass container, containing Ice?
Answer:
The water vapour present in air, comes in contact with the cold outer surface of the container and gets condensed. This causes formation of water droplets.
Question 3.
liquids and gases can be compressed hut it is difficult to compress solids. Why?
Answer:
Liquids and gases have large intermolecular space. Thus on applying pressure externally on them, the molecules can corne more close, thereby minimising the space between them. But in case of solids there is no intermolecular space to do so.
Question 4.
If you open a bottle of perfume in one comer of a room, it immediately spreads throughout the room. Explain the properly insols-ed.
Answer:
The property involved is diffusion. In a gas, the particles arc free to mese an a chaotic motion at a great speed throughout its containing vessel. Thus, when you opcn a bottle of perfume in one corner, the particles of the perfume move at random motion in all directions and mix with othergas particles in the air. Thus, they reach instantaneously to our nose.

Question 5.
Why do people perspire a lot on a hot humid day?
Answer:
On a humid day. due to the heat, our body starts sweating for thecooling mechanism by evaporation. However, the humid air cannot hold any more water on a humid das and therefore the rate of evaporation decreases. Hence. sweat or perspiration is seen.
Question 6.
Hoss does the rate of diffusion change with (a) density of liquid and (b) temperature? Gise examples.
Answer:
(a) Rate of diffusion decreases with density of s liquid. Honey is denser than ink. If you add a drop of ink and honey ‘n two separate jars filled with water, it will be ohscned that honey takes longer time to reach the bottom of the jar than by the ink.
(b) Diffision increases with rise of iempersture. For example, liquids mix faster at higher temperaiures.
Question 7.
How do you differentiate between solids, liquids and gases on the basis of their inciting poInts 2nd boiling points?
Answer:
- Solids: They hase melting and boiling points abose room temperature.
- Liquids: They hase melting pointa below room temperature and boiling point abose room temperature.
- Gases: They have both melting and boiling points below room temperature.
Question 8.
Which property of the gas is utilised when natural gas is supplied for vehicles?
Answer: A gas is highly compressible and a large quantity of it can be compressed to a small volume. Therefore, natural gas is compressed and is supplied for use by vehicles in the form ofCNG (Compressed Natural Gas).
Question 9.
On a hot das, why do people sprinkle waler on (be roof or open ground?
Answer:
On a bot das, the surface of roof or ground absorbs large amount of heat and remains bot. On sprinkling water on these surfaces, the water absorbs large amount of heai from 11w surface due to its high laient heat of sapsietsation and ewaporates. thereby allowing the hot surface to cool.
Question 10.
Esplain how dIffusion nf gases In water is essenilal?
Answer:
The gases from the atmosphere diffuse and dissolve in water. Difluasion of gases like oxygen and carbon dioxide ¡n water is essential for tIse survival ni aquatic animals and plants! Aninsals breathe – in this oxygen dissolved in watet foe their survival and planis can use carbon dioxide
dissolved in water foe photosynthesis.
Question 11.
Why are liqnids and gases called fluids?
Answer:
Liquids and gases can flow. Liquids can flow from higher pressure to lower pressure. The gases flow in all available directions. Due to this property of flowing, both of them are called fluids.
Question 12.
On a hot day, why do we feel pleasant sitting under a tree?
Answer:
Trees have a lot of leaves which constantly show transpiration. Transpiration is the loss of water through tiny pores of leaves called stomata. When this water comes on the surface of leaf the water evaporates, thereby causing a cooling effect. Therefore, we feel pleasant sitting under the tree on a hot sunny day.

Question 13.
How is the high compressibilit property of gas useful to us?
Answer:
The gases have high compressibility. This property is used in the following
situations:
- LPG is a fuel which is made up of petroleum gas. On compressing this petroleum gas. it forms liquid.
- Oxygen cylinders in the hospitals have compressed gas filled in it.
- CNG is a natural gas, which is compressed and used as a fuel in vehicles.
Question 14.
What ¡s pressure? What is its unit? On what factor does the pressure of a gas depend?
Answer:
The pressure exerted by a gas is the force exerted by gas particles per unit area on the walls of the container. It is expressed in pascal (Pa). I atmosphere 101325 x 10 Pa. The pressure depends on the average kinetic energy of the particles which depends on temperature of the gas.
Question 15.
How would you show that the three states of matter are interchangeable?
Answer:
On heating a solid substance and then cooling the vapour formed, we can show that the three states of matter arc interchangeable. Take ice cubes in a beaker and heat. Ice changes into water. On further heating, water changes into steam. On cooling, water vapour again changes into water and on further cooling water changes to ice.

Question 16.
Chee freezes at room temperature but mustard oil does not freeze even in winters. Which of these has a higher melting point and lower intermolecular forces?
Answer:
Ghee freezes at room temperature but mustard oil does not, it shows that intermolecular forces in ghee are stronger than those in mustard oil. The higher the intermolecular forces, the higher is the melting point. Therefore, ghce has a higher melting point than that of mustard oil.
Question 17.
Define evaporation.
Answer:
The phenomenon of change of a liquid into vapours at any temperature below its boiling point is called evaporation. Water, if left in an open vessel at room temperature, disappears after some time due to evaporation.
Question 18.
Is it true to say that fluorescent tubes contain only plasma?
Answer:
It is not correct to say that fluorescent tube contains only plasma. Inside a fluorescent tube there is helium gas or some other gas. The gas gets ionised or charged when electrical energy flows through it.
Analysing & Evaluating Questions
Question 19.
A sample of water under study was found to boil at 102°C at normal pressure. It the water pure? Will this water freeze at 0°C? Comment.
Answer:
(a) The given sample of water is not pure.
(b) The given sample of water will not freeze 0°C. The impurities raise the boiling point and lower the freezing point of water. Therefore, the given sample of water will freeze below 0°C.
Question 20.
A strong smelling gas ¡s stored in cylinders as liquid, but it comes out of the cylinder as gas.
(a) Name the gas.
(b) Vhat causes this change in state?
(c) Name the process of this conversion.
Answer:
(a) LPG (Liquefied Peroleum Gas).
(b) LPG gets converted into gas due to lowering of pressure.
(c) Vaporisation.
Long Answer Type Questions
Question 1.
Discuss the factors which affect evaporation.
Answer:
1. Surface area: Evaporation is a surface phenomenon. Thus, escaping of particles from liquid state to vapour state depends on surface area. Therefore, the rate of evaporation increases with surface area.
2. Temperature: If the temperature is increased, the rate of evaporation also increases. Due to increase in temperature, the particles gain more kinetic energy and change their phase from liquid to gaseous. Water will evaporate faster in sun than in shade.
3. Humidity: Humidity is the amount of water vapour present in the air. At a given temperature, air cannot hold more than a fixed amount of water vapour. Therefore, the rate of evaporation decreases with increase in the humidity of air.
4. Wind speed: With the increase in wind speed, the rate of evaporation increases. The particles of water vapour move away with the wind, decreasing the amount of water vapour in the surroundings.

Question 2.
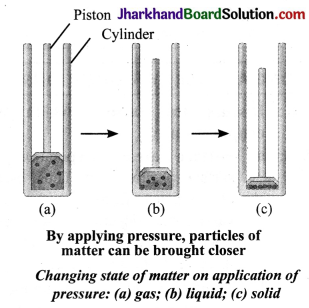
How are particles of matter affected with increasing or decreasing pressure on the matter at a given temperature?
Answer:
On increasing pressure, particles of a matter come closer and move apart when pressure is reduced at a given temperature. Thus, if pressure is increasingly applied on a gas, particles of the gas come closer and closer and eventually the gas may change into liquid and then into solid form.
Question 3.
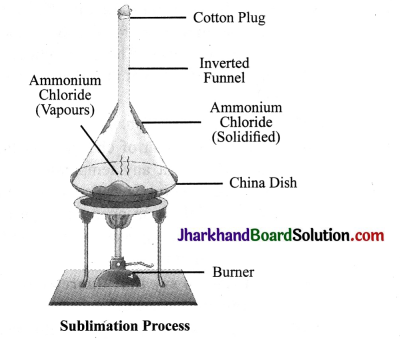
What is sublimation? With the help of an activity describe the sublimation of a solid substance. Name two substances used in our daily life that sublime on heating.
Answer:
We know that matter changes its state on heating from solid to liquid and from liquid to gas. However, there are some substances that change directly from solid state to gaseous state. This change of state from solid to gas without changing into liquid state is called sublimation. For example, camphor and ammonium chloride on heating change directly into vapour.
Activity
Take some ammonium chloride. Powder it and put in a china dish. Cover the china dish with an inverted funnel. Take some cotton and plug the stem of the funnel. Now, heat the china dish.
You will observe fine crystal particles depositing on the innerwalls ofthe funnel. Thus on heating, ammonium chloride is directly converted into ammonium chloride vapours which condense on the inner walls of the china dish.
Question 4.
Explain the diffusion of copper sulphate solution into water.
Answer:
The copper sulphate crystals dissolve slowly and form a layer of copper sulphate solution in the beaker. This blue copper sulphate solution diffuses into clear water and clear water diffuses down towards blue solution. This diffusion goes on until the whole water turns blue. So the spreading of blue colour is due to the diffusion of the blue copper sulphate solution and water into each other.