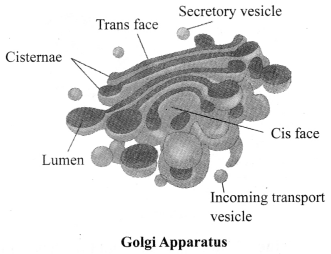
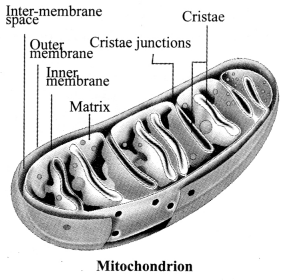
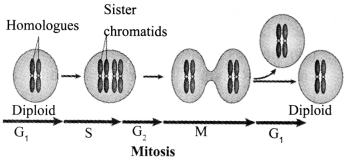
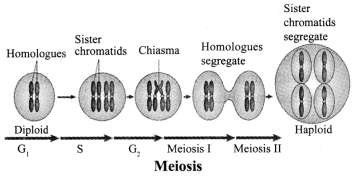
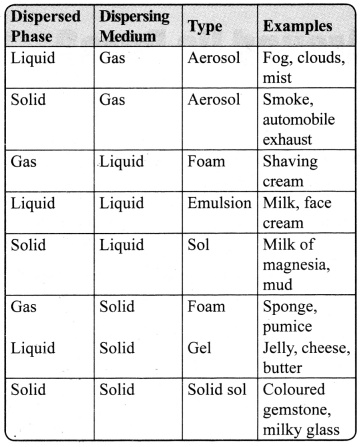
JAC Board Class 9th Science Chapter 6 Notes Tissues
→ A group of cells having a common origin and similar function are termed as tissues.
→ Plant tissues: They are primarily classified into two groups:
a. Meristematic tissues
b. Permanent tissues
Meristematic tissues: They are capable of dividing continuously to produce new cells. The meristematic tissues are present only at the growing regions such as shoot tip. root tip and at the base of intemodes and leaves.
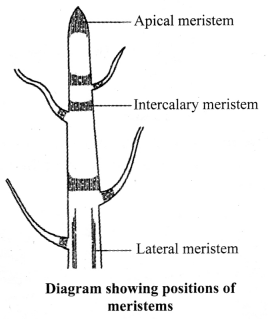
Depending on the area where they are present, meristematic tissues are classified as apical, lateral and intercalary. Meristematic tissues are very active, have dense cytoplasm, thin cellulosic walls and prominent nuclei. They lack vacuoles.
![]()
Permanent tissues: Formed from meristematic tissues, the cells in the tissue lose the ability to divide. They have differentiated and attained a permanent shape suitable for their functions. Permanent tissues are divided into two categories. Simple permanent tissues: Tissues which are made up of only one type of cells are called simple tissues.
Parenchyma, collenchyma and sclerenchyma are examples of simple tissues,
a. Parenchyma: Composed of unspecialised cells with relatively thin cell walls, large intercellular space, present in soft parts of the plant. Their main function is storage.
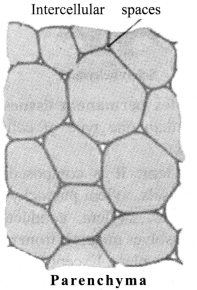
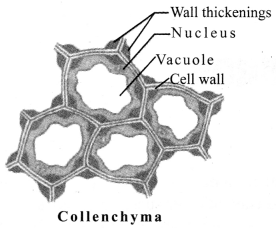
b. Collenchyma: It is composed of living and elongated cells with cell walls and irregularly thickened at the comers. There is very little intercellular space. It provides mechanical support and elasticity to plant. It helps in bending of leaves and stems.
c. Sclerenchyma: It is composed of long, narrow and thick-walled cells. This tissue is made up of dead cells, and there are no intercellular spaces. Sclerenchyma cells are dead. They are present in seeds, nuts, husk of coconut- fibre of jute, etc.
![]()
→ Complex permanent tissues: Made up of more than one type of cells (conducting tissues).
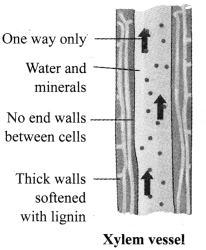
a. Xylem: It is composed of tracheids, vessels, xylem parenchyma and xylem fibres. Xylem conducts water and dissolves minerals from roots to all parts of the plant. Except xylem parenchyma, xylem cells are dead cells.
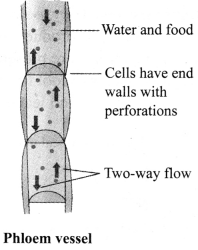
b. Phloem: It conducts food from the green leaves/parts to other parts of the plant. It is composed of four elements-sieve tubes, companion cells, phloem parenchyma and phloem fibres. Except phloem fibres, phloem cells are living cells.
→ Animal tissues: Animal tissues are of four types in higher animals including human beings.
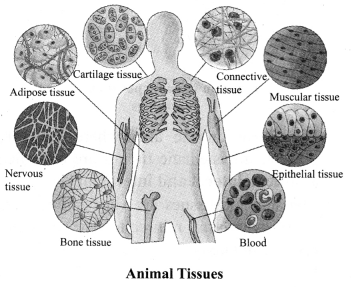
a. Epithelial tissues: They are present for the covering of the external surfaces, internal cavities and organs of the animal body. Various types of epithelial tissues are:
- Squamous epithelium in the lining of mouth and oesophagus.
- Cuboidal epithelium in the lining of kidney tubules and salivary glands.
- Columnar epithelium in the intestine and columnar epithelium with cilia in the lining of respiratory tract.
- Glandular epithelium in the glands aids in a special function as gland cells, which can secrete at the epithelial surface.
b. Muscular tissues: They are made up of muscle cells, called muscle fibres. There are three types of muscle fibres:
- Striated muscles (skeletal muscles or voluntary muscles): Cells are cylindrical, unbranched and multinucleate.
- Smooth muscles (involuntary muscles): Cells are long, spindle-shaped and possess a single nucleus.
- Cardiac muscles (involuntary muscles): Cells are cylindrical, branched and uninucleate.
c. Connective tissues: They connect various tissues and organs. They provide support to different parts of the body by forming packaging around different organs of the body. The different types of connective tissues in our body are bone, cartilage, tendon, ligament and blood.
d. Nervous tissues: The tissue responds to stimuli. The brain, spinal cord and nerves are composed of nervous tissues or neurons. A neuron consists of cell body, cytoplasm, nucleus, dendrite, axon and nerve ending. The neuron impulse allows us to move our muscles when we want to respond to stimuli.